Dr. Paul Volden recently presented a
webinar on
humanized immune system mouse models, focusing on engraftment of PBMCs and isolated cell subsets. Taconic has extensive experience with humanized mice, both from internal research as well as many collaborations with external partners. Although hematopoietic stem cell-engrafted models such as the
huNOG and
huNOG-EXL may be better known, these alternate humanization approaches offer advantages for certain studies.
We present the full webinar Q&A here.
Graft-vs-Host Disease
Q. What is graft vs. host disease (GvHD) and how is GvHD relevant to mouse models with human immune systems (HIS)?
A: GvHD occurs when engrafted immune cells recognize their host as non-self and "attack" using immune responses that are meant, under normal immune conditions, to protect against foreign invaders (e.g. bacteria and viruses). GvHD can occur in patients that undergo immune cell transplant and, if not adequately managed, can be lethal. Similarly, human immune cells can mediate GvHD when engrafted in immunodeficient murine hosts. Depending on host properties (e.g. level of immunodeficiency) and properties of engrafted cells (cell type(s), cell maturity, quantity engrafted, etc.), the onset and severity of GvHD in murine systems can be tuned to benefit research objectives. Indeed, mouse models of GvHD are frequently leveraged to study autoimmune disease, dysregulated immunity, and therapies meant to intervene during deleterious immune responses. In many HIS model applications, GvHD occurrence can negatively impact a researcher's ability to interpret key study readouts, or more generally impose limitations on the overall study. For example, GvHD onset can be the key determinant for study window duration. Researchers intending to leverage HIS models should determine whether, what, and how aspects of GvHD can present in the models being evaluated and prior to study design.
HSC-engrafted models
Q. HSC-engrafted models require 10-12 weeks for human CD34+ stem cells to engraft and differentiate. Why doesn't this model develop graft vs. host disease during that time?
A: It helps to contrast the
CD34+-engrafted model to the
PBMC-engrafted model. In the PBMC-engrafted model, you have mature T cells amongst those PBMCs. Those T cells developed in a human and were selected in a human thymus, selected to understand self from non-self within the human context. Then you engraft them into a mouse, and those T cells see non-self. In that setting, it's easy to understand why GvHD occurs and why it occurs so rapidly. In the CD34-engrafted model, the hematopoietic stem cells initially home to the bone marrow and hematopoiesis takes place. You can find human T cells in the mouse thymus. The developmental process that takes place within the host provides the explanation. The T cells which develop within the mouse host are to some degree trained to recognize the mouse as self. Those T cells don't function completely normally as in a human immune system, but they are developing within a mouse host.
Q. What humanization percentage is obtained in mice after engraftment with human HSC cells?
A: It depends on the specific host that is engrafted, the quality and type of HSCs, and the engraftment protocol. Generally, a more immunodeficient host will engraft at a higher percentage than a similar host that is less immunodeficient. Engraftment efficiency can also be increased in hosts that express human proteins, as is observed in NOG-EXL mice through expression of two human cytokines. Comparing CD34+ HSCs from umbilical cord vs. from bone marrow aspirate illustrates how HSC type can influence engraftment.
NOG mice engrafted with umbilical cord CD34+ HSCs develop an immune system replete with human B cells and T cells and around 30-60% peripheral blood hCD45 chimerism by 12-16 weeks post engraftment. The same humanization protocol using CD34+ HSCs from bone marrow aspirates would deliver few or no animals with greater than 25% hCD45 in peripheral blood and few or no animals showing any degree of human T cells.
PBMC engraftment
Q. Can PBMCs be injected via the intraperitoneal (ip) route or is intravenous (iv) injection always better?
A: You can inject PBMCs ip. There are published reports that suggest iv delivery of PBMCs is more efficient, and I typically advise as if this were generally true. But there is preclinical data where both routes were evaluated, iv versus ip, and in one specific case that comes to mind, the results showed that ip was better. Better within the context of the particular experimental goals. I'm hesitant to say firmly that either iv or ip is better in all cases. The best route is determined empirically. Evaluate the two routes prior to starting your planned study and judge based on your specific experimental needs.
Q. While many studies use iv systemic injection of PBMCs, some studies utilize direct injection of human PBMCs into an engrafted tumor. Is one approach better to use depending on circumstances?
A: This refers to co-engraftment. It's a different approach. With co-engraftment, you are forcing an interaction between the immune cells and the tumor. Why would you want to do that? It's quick. You generally don't have to worry about GvHD because you're using far fewer PBMCs and you are localizing them to the site of the tumor. What's really cool about this approach from an experimentalist's perspective is that you can play around with it. There are a lot of different things you can do. You can add in antigen-presenting cells and localize them within the tumor or localize other human immune cells that aren't supported by the host to get readouts for short term studies. All those options are great, but there are drawbacks. You need to decide if it's the right approach for your question. Co-engraftment studies are recognized as more useful for mechanistic studies, whereas the systemic PBMC engraftment approach is probably the better efficacy model. Better efficacy model in what way? Recall the huPBMC-NOG work from Numab and Pieris presented earlier; the data suggests their drugs have an improved safety profile. They were not activating T cells in the periphery. That is a very important insight that you can't get from co-engraftment experiments. It requires the interaction of the therapeutic with T cells in the periphery in order to make that observation. Simultaneously, if you are systemically injecting PBMCs, now you are able to judge your drug's ability to modulate the migration of the effector cell population that you're engaging in the periphery into the tumor. You can't do that with co-engraftment.
Q. Are there ways to enhance PBMC engraftment?
A: Preconditioning, using irradiation or busulfan and other chemical agents, tends to enhance the engraftment of human immune cells. Dosage is important for both approaches. It's also important for researchers to understand that enhancing human PBMC engraftment will likely also mean accelerating the onset of GvHD.
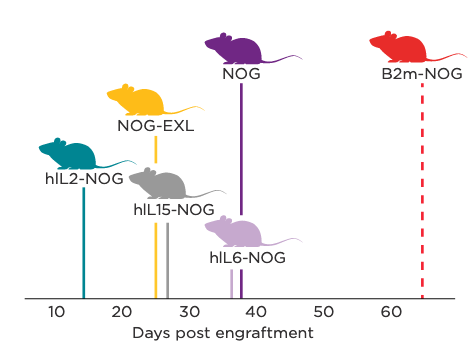
Graphical representation of terminal-stage GvHD onset in NOG Portfolio models following human PBMC engraftment.
Q. Please elaborate on the benefits of banking PBMCs and other human cells for engraftment. Other than variability concerns, isn't it a good idea to use cells from various different donors?
A: Let's talk about variability. This is an important consideration for all humanized models, including both PBMC- and HSC-engrafted. Donor-specific variability can and will affect PBMC engraftment kinetics, including the timing of GvHD onset. Those properties dictate the length of time you can keep the animals on study. It's important to have that variability under control. Losing key data points because you have earlier mortality than expected can ruin a study. That's something you can de-risk by pre-evaluating those PBMCs
in vivo and then banking them. That's a clear benefit to PBMC banking. It's also a good idea to use cells from several donors, and you can do this by banking cells from multiple donors. Think about this if you're a basic researcher: let's say you've got PBMC cells banked and you see a donor-specific effect. You want to know how and why, right? You can go back to those banked cells to investigate that basic question. In the preclinical space, banking evaluated cells becomes even more important and powerful. Modern preclinical drug development takes place globally, collaboratively and increasingly, through multiple CRO partners. Spreading related work across more than one location introduces variability, probably more variability than almost anything else you could introduce into your work. You can't really control that unless you decide you're going to be the one person in preclinical development that isn't going to work collaboratively and doesn't work with CROs. What you can do is control the raw materials like the specific kits used across sites, the cell lines, or the immune cells. That's the power and benefit of banking those PBMCs. You may have to change the study location, but you can use the same PBMCs. Contrast that with the CD34+ perspective: you need three months or more to be able to prescreen any donor, so it's really not practical. Even if you did prescreen and you found a donor you liked, you're only going to get one, maybe two studies if you're lucky from the cells you have. So the PBMC volume, banking, and ability to return to a donor in the freezer gives you a ton of power. That's why we are very excited about what we've been working on related to huNOG-EXL and being able to return to donor HSC cells with that model.
Other HIS Model Applications
Q. The research reviewed in the webinar covered biologics. Can these models be used to study small molecule drugs?
A: Absolutely. There is a bias towards biologics because of the growth in cancer immunotherapy, which has become populated with more and more biologics of increasing complexity. There are differences between biologic and small molecule test articles. If you have questions about how to assess small molecules in humanized models, I encourage you to reach out to Taconic to set up a consultation with one of our scientists.
Q. Transgenic expression of human cytokines in base immunodeficient models is one approach to supporting engraftment and differentiation of particular human immune cell types. Hydrodynamic gene delivery is another approach to get expression of human cytokines in host strains. What are the pros and cons of each approach?
A: The clearest difference in an experimental setting is ease of use. You have options for introduction of human cytokines to support particular human immune cells. If you use a transgenic model, things just got a lot easier. You don't need the expertise to perform a hydrodynamic delivery of plasmids that express the cytokine. In most experimental settings, you don't just inject that plasmid once, you have to perform multiple injections. That's resources, time and labor. So there are technical challenges, there can be higher resource demands, but there are benefits as well. You get versatility. You can introduce whatever cytokine you want. Maybe you can tease the system to support certain cell types that you want. So for basic research, hydrodynamic gene delivery offers some interesting and compelling advantages. In the preclinical setting, you might get pretty robust models established with gene delivery of a single cytokine, like hIL-15, but what happens when you need more cytokines? For example, can you emulate NOG-EXL, which has both human GM-CSF and IL-3? I would project it's going to be far more difficult because with the amount of variability with those injections, things are going to get way more complicated if you're trying to modulate expression levels so you can get a NOG-EXL type of performance. You have to tune two different cytokines with hydrodynamic gene delivery.
Q. Are there any models that weren't mentioned in the webinar which display good reconstitution of human T cells and human NK cells?
A: Yes. The best way to address this is to have a scientist to scientist discussion. I can probe what you mean by "good" NK cell engraftment. There are other models out there which support human NK cells. And different approaches, such as hydrodynamic gene delivery which can support human NK cells
in vivo. But what you want is far more than for those cells to just be there. You need those cells to be both present and functional. Cells with perforin and granzyme levels that are adequate, cells that can mediate ADCC, cells that have killer immunoglobulin receptors that function when they see absence of MHC class I. These are the details to dig into when you're looking for "good" engraftment, and these are the kinds of details we can discuss when you consult with a Taconic scientist. We can tell you about other models which support human NK cells, other approaches, including models available outside of Taconic's portfolio. They can discuss the strengths of limitations of the different models and approaches as they apply to your specific experimental question and the type of drug you are investigating. We can help you out.
Q. Are humanized mice leveraged that use immune cells coming from different clinical conditions?
A: Yes, they are. Within the webinar I briefly discussed how the hIL-2 NOG model facilitated reproducing clinical responses. The clinical responses that were reproduced were complete anti-tumor responses to adoptively transferred T cells, and these transferred T cells were isolated from the very same patient tumors that were established as PDX in hIL-2 NOG mice. Other examples from various disease settings have also been described in the literature.
Q. How do sex differences in the engrafted cells or across the engrafted host impact humanization?
A: I am not currently aware of literature describing significant differences from the immune cell donor's sex on engraftment properties. With respect to the host, whether and to what extent mouse sex impacts engraftment for the full spectrum of commonly engrafted human immune cells isn't known. However, we and others have observed a slightly lower peripheral blood chimerism in HSC-engrafted male mice. Some research suggests androgens are immunosuppressive. This could provide most or part of the explanation for slightly decreased engraftment efficiency in male vs. female NOG mice injected with human HSCs.
A: We have not worked with NOG mice expressing both hIL-15 and hIL-2, but our colleagues from the Central Institute of Experimental Animals have made this model. It played a minor role in one of their recent publications titled
Long-term maintenance of peripheral blood derived human NK cells in a novel human IL-15- transgenic NOG mouse.
 Read the Related Taconic Biosciences' Insights:
Read the Related Taconic Biosciences' Insights:
 Download the Related Taconic Biosciences' White Papers:
Download the Related Taconic Biosciences' White Papers:







.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)
.jpg)


.jpg)



.jpg)




.jpg)

.jpg)
.jpg)






