The immune system is a complex orchestration of blood and tissue cells that protect us from pathogenic invaders like bacteria, and viruses. Our knowledge of the immune system has grown exponentially over the past twenty five years, with a multitude of discoveries originating from studies in wild type and genetically engineered mouse models. Among them is a pivotal role for the immune system to protect against cancer. Immuno-oncology is aimed at understanding the intricate components of the immune system that are able to detect and kill abnormally dividing cells before they develop into tumors. More recent evidence suggests nearly all cancers employ one or more biological counter-measures to effectively hide from the immune system and elude detection. Because of this, researchers are now investigating strategies to awaken a patients' own immune system against their cancer.
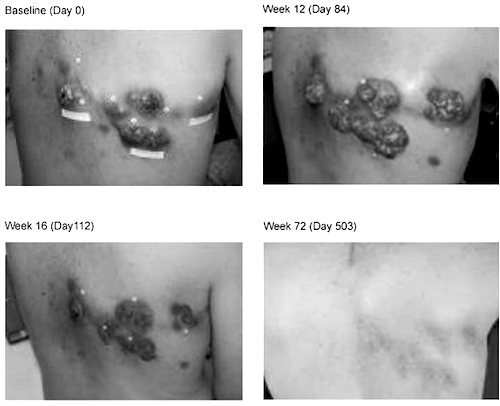
The past decade has seen remarkable progress in Immuno-oncology; leading to a series of blockbuster new drugs for the treatment of stage IV melanoma. In 2011, the FDA approved a new treatment called Yervoy®, released by Bristol Myers Squibb, which targets one such counter-measure, the immune checkpoint blockade molecule called CTLA-4. In a recent follow up report on the first patients treated with Yervoy®, the clinicians reported 20-30% of patients treated responded with partial or complete responses, with extended lifespan of a median 14 months, and five year survival rates at about 20%. 14 of 15 "complete responders" are going on 54+ and 99+ months post treatment without recurrence. The clinical success was so remarkable that the FDA rushed approval of Yervoy® for this previously untreatable condition. This image is taken from the first publication describing the clinical findings, and shows a patient with intractable stage IV melanoma. At 72 weeks post treatment with Yervoy®, the superficial skin tumors are completely resolved and the patient is without disease. The clinicians in charge of following the first group of patients treated with the drug describe this treatment as "potentially curative"; a language choice rarely used in the oncology setting. This September, Merck won approval for Keytruda® another immuno-oncology drug aimed at the molecule PD-1, which has garnered similar success profiles in patients that did not respond to Yervoy® treatment. Importantly Keytruda® recently won blockbuster designation by the FDA for a second indication, non-small cell lung cancer.
Undoubtedly, the pharmaceutical industry is in a major race to develop drugs aimed at these molecular targets. Since Yervoy's release, at least 10 major pharma and biotech companies, and numerous start-ups have pushed forward with immuno-oncology drug development programs. What's more, the biology behind immuno-oncology drugs strongly suggests they will be even more effective as combinatorial therapies with one another, or with conventional small molecule drugs. As well, there is additional increasing pressure to test them for efficacy against many other types of cancer including kidney, lymphoma, lung and liver.
Humanized immune system mice usher in a new era of drug discovery
Yet with all of the initiative to rapidly develop this new class of drugs for many different types of cancers, major challenges remain. Species differences between mouse and humans prevent testing for efficacy in traditional animal models. Sensing this growing need for better models, Taconic Biosciences has endeavored to make commercial production of "immune system humanized mice" based on the
CIEA NOG mouse®. In 2014, the preclinical research models and services group, led by Dr. Leon Hall, introduced the
huNOG mouse model.
What are Humanized Immune System aka "huNOG" Mice?
Immune system humanization is a process where human hematopoietic stem cells are injected into a juvenile immune-deficient mouse (the
CIEA NOG mouse®). The NOG mouse does not have a fully functioning immune system, and the injected hematopoietic stem cells are able to differentiate into multiple critical immune cell types with similar functional characteristics of their native human counterparts. The
huNOG mice are generated in sufficient quantities to enable routine testing of drugs designed to activate the human immune system against different types of cancer. In addition to the huNOG mouse model, the immune system humanization program offers two additional engraftment strategies, each designed to address the discrete needs of research programs interested in drug design for human immune cell activation. Thus, huNOG and the other humanized immune system models are exciting new products offered by Taconic with critical significance to the research programs of companies investigating new immuno-oncology therapies.
In summary, the changing landscape of pharmaceutical and biological drug development is driving the need for newer, better models of human physiology. Taconic Biosciences' program in immune system humanization is poised to meet this need and therefore enable the discovery of the next generation of cancer treatments for all.
 View the Humanized Mice White Papers:
View the Humanized Mice White Papers:
Image courtesy of: Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010 Sep 22;102(18):1388-97. doi: 10.1093/jnci/djq310. Epub 2010 Sep 8. Review. PubMed PMID: 20826737; PubMed Central PMCID: PMC2943524.

 View the Humanized Mice White Papers:
View the Humanized Mice White Papers:

 View the Humanized Mice White Papers:
View the Humanized Mice White Papers: