2020
 When we look back on 2020, how will we remember it? Will we look back on it as a year in which many people were affected by COVID-19? Certainly. We offer another perspective here: how the life sciences community stepped up to do battle with COVID-19.
When we look back on 2020, how will we remember it? Will we look back on it as a year in which many people were affected by COVID-19? Certainly. We offer another perspective here: how the life sciences community stepped up to do battle with COVID-19. What began as an ordinary year headed downhill as we learned more of an emerging viral outbreak. SARS-CoV-2 was first observed in Wuhan China as early as November 2019 and within a matter of months, spread to all parts of the world. As cases began to emerge worldwide, we learned more about the virus and its pathogenesis. However, it would be many months before we understood how the virus spread and what measures might be effective in reducing the spread. Many ideas arose on how the virus might spread. Could the virus live on surfaces for prolonged periods of time? How long was a person positive for the virus infectious? We now have a better understanding that the virus spreads primarily from person to person when in close contact. Social distancing and wearing a mask have proved effective in diminishing spread.
Another aspect of COVID-19 was the sheer number of symptoms caused by infection and how deadly it was. When compared to the seasonal flu (influenza virus), the SARS-CoV-2 virus has a death rate 52 times higher. Only 0.1% of those who contract the flu die, while 5.2% of those who contract COVID-19 die (US Centers for Disease Control and Prevention).
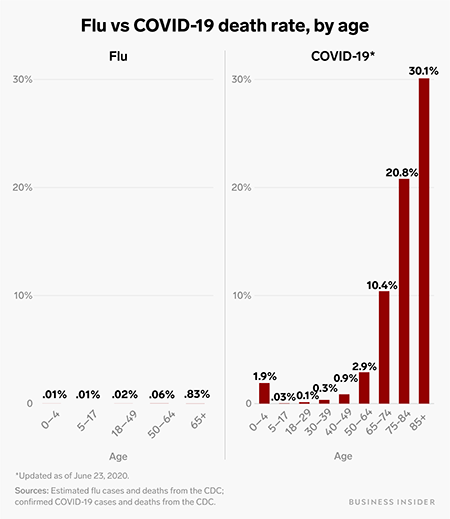
Flu vs. COVID-19 death rate, by age. Source: Science Alert
The economic impact is less easy to understand. While stock markets around the world took a hit, they quickly recovered. Some industries are faring well, especially those with an online presence. Brick and mortar stores have suffered. Many lost jobs, and while governments were quick to pass economic stimulus packages propping up the economy, many of those programs are sun setting, leaving many struggling to make ends meet.
As with any issue, large or small, there are lessons learned. COVID-19 is no different. Once the gravity of the situation was realized, people from all walks of life responded. People reached out to neighbors. Many retail stores learned how to go online with their products and provide curbside touchless pickup. Libraries, long a building that people visited, learned how to deliver and return books in a manner that reduced the risk of infection.
And how did the life sciences community respond to COVID-19? The community rallied together, leading to a robust program to understand and battle COVID-19. While borders closed, life science opened channels of communication. There is no doubt that the life sciences community offered hope and help to people around the globe.
Here we present a short synopsis of the response.
Knowledge
A few institutions jumped to the forefront in collecting, analyzing, and presenting COVID-19 related information. A few of these include Johns Hopkins Coronavirus Resource Center, European Centre for Disease Prevention and Control, and the World Health Organization. These organizations strived to collect accurate data and present it in ways that could help countries, businesses, and individuals make decisions. This pooling of data is a tremendous resource for scientists who are conducting epidemiological studies.Publishers strove to get COVID-19 research papers out as quickly as possible. This allowed a rapid transmission of information, expediting the discovery process. A PubMed search shows 92,437 publications related to coronavirus (Search "COVID-19 or coronavirus or SARS-CoV-2"). 77,128 (83.4%) of those publications have been released in 2020. This underscores the level of effort researchers across the world have taken to help us understand, treat, and prevent the spread of the SARs-CoV-2.
In an early 2020 publication, investigators in the Modern Virology Research Center at Wuhan University identified the virus as a novel human coronavirus designated as SARS-CoV-2 (Chen, 2020). This novel virus is related to the SARS-CoV virus identified in a pandemic in 2003. However, SARS-CoV did not persist in the population and its worldwide effect was minimal when compared to SARS-CoV-2. For a concise comparison of both viruses, see: How does SARS-CoV-2 compare to SARs-CoV-1?.
Diagnosis
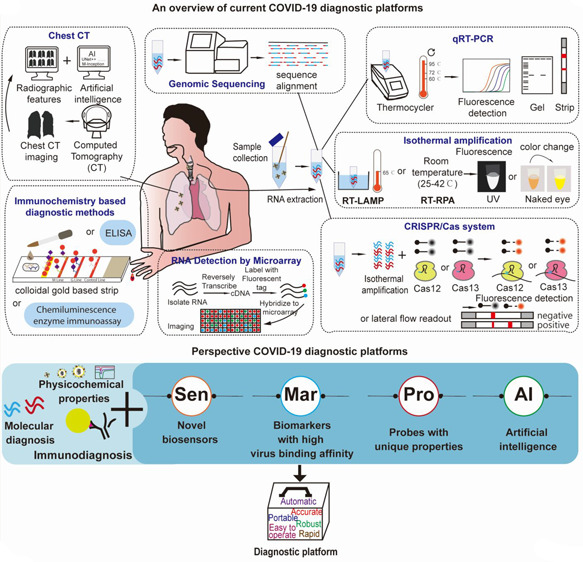
Current and Perspective Diagnostic Techniques for COVID-19. Source: ACS Infect Dis.
Treatment
Developing detection methods for this novel coronavirus was relatively straightforward. Treatment, however, has been more of a challenge. While primarily a respiratory virus, COVID-19 affects multiple organ systems, thus making it difficult to treat.Those infected with COVID-19 present a range of symptoms, including being asymptomatic. Data has shown that these symptoms appear 2-14 days after exposure to the virus.
- Fever or chills
- Cough
- Shortness of breath or difficulty breathing
- Fatigue
- Muscle or body aches
- Headache
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
- Diarrhea
Understanding the importance of moving treatment into the clinic, the FDA created the Coronavirus Treatment Acceleration Program (CTAP). This emergency program aims to help expedite the process of testing and approval without bypassing critical safety testing or clinical trials. The CTAP dashboard shows over 550 drugs in the planning phase, 350 trials reviewed, and 5 treatments gaining authorization for Emergency Use. The treatment modalities include antiviral agents, cell and gene therapies, immunomodulators, neutralizing antibodies, and combinations.
Prevention
Prevention falls into two categories: First, how to prevent or slow down the spread of the disease, and second, how to prevent the disease from infecting more of the population through vaccination:- The most effective means of preventing spread between people without a vaccine appears to be wearing a mask coupled with social distancing, which reduces the spread of viral particles. While these methods are effective, they have substantial emotional, social, and economic impacts. A recent article, Face masks: what the data say published in Nature online, examines the data on how effective masks are in reducing the spread of coronavirus. "...the science supports using masks, with recent studies suggesting that they could save lives in different ways: research shows that they cut down the chances of both transmitting and catching the coronavirus, and some studies hint that masks might reduce the severity of infection if people do contract the disease."
- While these physical methods are useful, an effective vaccine would greatly reduce the number of people becoming infected and help the return to pre-COVID normalcy.
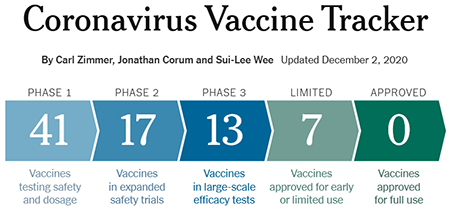
Coronavirus Vaccine Tracker. Source: New York Times
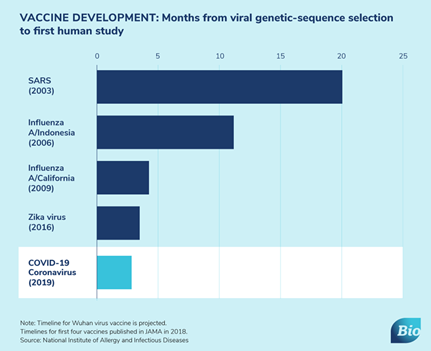
While early data show vaccines can elicit an antibody response, it is too early to know if they develop a robust response to confer protection from COVID-19 infection. The graph below shows how vaccine development is going when compared to previous viral outbreaks. Once developed, vaccines need to be manufactured, tested, and distributed. Given the urgency in vaccinating a large portion of the population, it will take a concerted effort on governments, companies, and private & public health organizations to accomplish the feat of administering hundreds of millions of doses. How the US might distribute a coronavirus vaccine provides an example of one country's plan to distribute vaccines.
Research
Source: BIO
However, one missing resource was a mouse model designed specifically for COVID-19 research. Models designed and generated for SARS-CoV could be used to study the SARS-CoV-2 virus, but they were not readily available. Realizing time was of the essence, Taconic reached out to Dr. Chien-Te Tseng at the University of Texas Medical Branch and began working immediately to import the hACE2 mouse. Through RapidEXPANSION™, cohorts of mice became available in October and were immediately shipped to investigators.
An August 2020 publication titled: Animal and translational models of SARS-CoV-2 infection and COVID-19 (Johansen, 2020) offers an extensive review of models used in coronavirus research. The table below examines Genetically Engineered Models (GEMs) used in COVID-19 research. While not extensive, these models provide a resource to help better understand SARS-CoV-2 infection and resulting COVID-19 disease.
Summary of available mouse models used to examine SARS-CoV-2 pathogenesis
| Mouse line | Promoter | hACE2 expression and localization | Infectious doses | Disease outcome |
| hACE2-HB-01 | mACE2 promoter | High expression in intestine and kidney. Moderate expression in heart. Low expression in lungs. | Up to 1 × 106 TCID50 | Mice lost ~10% body weight but all recovered. High viral titres in the lung, no obvious clinical signs. No morbidity. |
| HFH4-hACE2 | Forkhead transcription factor (HFH4/FOXJ1) | High expression in lungs, gut and brain. Low expression in liver and kidneys. | 3 × 104 PFU | Proportion of mice lost >20% body weight and died. Moribund mice had neutrophilia and severe lung damage evidenced by histopathology. |
| K18-hACE2 | Cytokeratin 18 (K18) High expression in lungs and colon. Moderate expression in small intestine, spleen, kidney, liver and heart. Low expression in brain. | 8 × 104 TCID50 | ~10% weight loss and symptomatic disease by 5 dpi. High viral titres and inflammatory cell counts in lungs. Extensive lung inflammation and histopathology. | |
| Up to 2 × 104 PFU | ~30% weight loss by 7 dpi. High viral lung titres by 3 dpi. Extensive lung and brain inflammation and histopathology. | |||
| 2.5 × 104 PFU | ~25% weight loss by 7 dpi. High viral titres in the lungs, and modest viral titres in the heart, brain, kidney and spleen from 2 dpi. Reduced respiratory capacity from 7 dpi. RNA-seq of infected lung tissue shows high upregulation for innate immune response pathways. | |||
| 1 × 105 PFU | ~20% weight loss by 5 dpi, with uniform mortality by 6 dpi. High viral titres in the lung from 2 dpi, as well as moderate viral titres in the nasal turbinates and brain from 2-4 dpi. Cytokine storm observed in lungs and spleen from 2 dpi. | |||
| AdV-hACE2 | HFH4 under control of cytomegalovirus promoter in incompetent adenoviral vector | High expression in lungs. Expression in other tissues not reported. | 105 FFU | Mice lost ~10% body weight over 8 days. High viral lung titres reported. No reported mortality. |
| Humanized ACE2 | Native mACE2 promoter | High expression in lungs, small intestine, spleen and kidney. Low expression in brain, ovary and heart. | 4 × 105 PFU | Mice had high viral titres in brain, trachea and lung. Aged mice lost 10% body weight. No obvious clinical signs. |
Summary
Returning to the Dickens' quote, "It was the best of times, it was the worst of times, it was the age of wisdom...". 2020 may be viewed as one of the worst times in modern history. Nevertheless, we also must view 2020 through the lens of how our life sciences community came together, leading to an "age of wisdom." Yes, there is still much work to be done, but the life sciences community deserves a big "Thank You" for their efforts in battling COVID-19.Explore more about Taconic's:
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)



