 Immuno-oncology researchers are looking beyond T cells to other immune cells for the next cancer breakthrough. Myeloid cells (cells that originate from the common myeloid progenitor derived from hematopoietic stem cells in the bone marrow) are among those new targets. This class of immune cells includes monocyte and granulocyte lineages including macrophages, dendritic cells, mast cells, basophils, eosinophils, and neutrophils. Myeloid cells have an important role in the innate immune response and also serve an immune regulatory function. In cancer, myeloid cells can either contribute to an immunosuppressive tumor environment or induce/enhance an anti-tumor immune response, which makes them ripe for manipulation for therapeutic ends. Researchers are investigating a number of different mechanistic strategies for immunotherapy involving myeloid cells, including immune checkpoint inhibition, cancer vaccines, adoptive cell transfer, and more.
Immuno-oncology researchers are looking beyond T cells to other immune cells for the next cancer breakthrough. Myeloid cells (cells that originate from the common myeloid progenitor derived from hematopoietic stem cells in the bone marrow) are among those new targets. This class of immune cells includes monocyte and granulocyte lineages including macrophages, dendritic cells, mast cells, basophils, eosinophils, and neutrophils. Myeloid cells have an important role in the innate immune response and also serve an immune regulatory function. In cancer, myeloid cells can either contribute to an immunosuppressive tumor environment or induce/enhance an anti-tumor immune response, which makes them ripe for manipulation for therapeutic ends. Researchers are investigating a number of different mechanistic strategies for immunotherapy involving myeloid cells, including immune checkpoint inhibition, cancer vaccines, adoptive cell transfer, and more.
Checkpoint inhibition in the myeloid realm
Checkpoint inhibitors have been quite successful for activation of anti-tumor T cell responses, and this same approach is being applied to myeloid cells. Checkpoints being explored include:- CD47-SIRPα interactions between CD47 expressed on tumors and SIRPα on macrophages and other myeloid cells. This interaction sends a "don't eat me" signal which prevents destruction of therapeutic antibody-bound tumor cells by macrophages and granulocytes. Disruption of this signaling pathway may be an effective way to enhance efficacy of antibody-based anticancer agents1,2.
- c-Rel is involved in development and function of myeloid-derived suppressor cells (MDSCs), which inhibit anti-tumor responses by the immune system. Inhibition of c-Rel may help dampen the immunosuppressive actions of MDSCs in cancer3.
- LILRB3 is a member of the human leukocyte immunoglobulin (Ig)-like receptor (LILR) family which is expressed primarily on myeloid cells. A proof of concept study in humanized immune system mice (used because mice do not express this receptor) demonstrated tumor tolerance in the presence of LILRB3 agonism. Thus, the authors concluded that LILRB3 has a powerful immunosuppressive effect, plays an important role as a myeloid checkpoint receptor, and its modulation could provide for advancement in tumor evasion and Immuno-oncology therapy advancements4.
Dendritic cells bridge innate and adaptive immunity
Conventional dendritic cells (cDCs) have important roles in anti-tumor immunity through their primary role for antigen presentation to the adaptive immune system. In particular, cDCs have a critical role in the T cell activation pathway and the recruitment/infiltration of activated T cells to tumor sites. Given these two immunostimulatory roles, cDCs can promote a response to anti-tumor immunotherapy. However, in contrast to these cDC functions, dendritic cells (specifically plasmacytoid dendritic cells, pDCs) can have an immunosuppressive role and the presence of pDCs in tumors is associated with poorer outcomes in human cancer.Approaches to targeting DCs involve enhancing DC maturation, quantities and function, increasing frequency of DCs in tumors and disrupting immunosuppression of DCs5,6. DC vaccination combined with chimeric antigen receptor (CAR) T cell (CAR-T) therapy is currently being investigated in clinical trials , and retroviral transduction of human CD34+ hematopoietic stem cells to generate myeloid cells bearing CARs has been explored7.
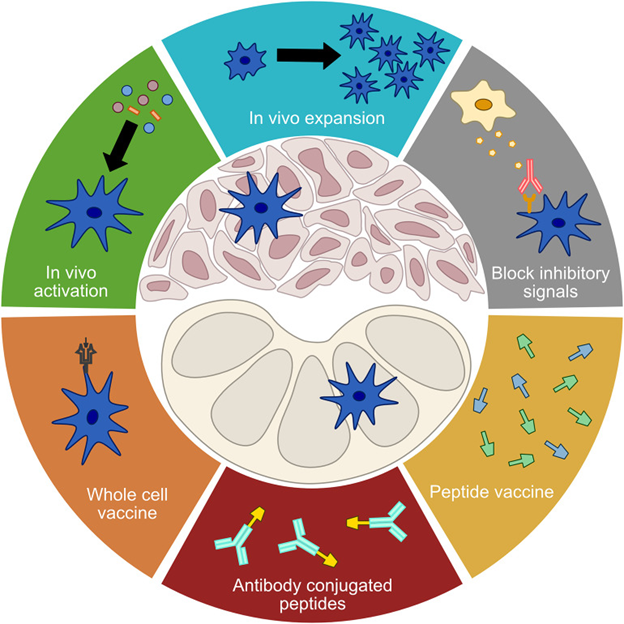
Figure 1: Approaches to manipulation of dendritic cells for immunotherapy. From Gardner et al. Distributed under the Creative Commons Attribution License (CC BY)8.
Macrophages
Macrophages are commonly found in tumors (tumor-associated macrophages or TAMs), and they are heterogeneous in origin and activation state. They can be either pro- or anti-tumorigenic, however, typically high levels of TAMs are associated with poor clinical outcomes in many tumor types. Approaches to the manipulation of TAMs include suppressing the creation of TAMs from circulating monocytes and converting existing TAMs to an anti-tumorigenic state5. Efficacy of CAR-bearing macrophages was evaluated in preclinical studies using HIS mice capable of supporting the reconstitution of human myeloid cells. In these HIS mice, tumor inoculation and subsequent dosing with CAR-transduced macrophages prolonged survival via the recruitment and interactions of these pro-inflammatory CAR macrophages and the tumor microenvironment9. This therapeutic approach has recently advanced to clinical trials for treatment of solid tumors.Neutrophils
Neutrophils are abundant and heterogeneous, with a primary function as first-line in the defense of the host against infection. However, neutrophils can also be associated with tumors (tumor-associated neutrophils, or TANs) with either a pro- or anti-tumor phenotype (as previously seen with many myeloid-based cells). Targeting strategies for TANs include targeting both the recruitment and polarization state (bilateral movement from pro- to anti-inflammatory) of these cells10. Adjuvant treatment of melanoma patients with type I interferon prevents neutrophil migration from the bone marrow, reducing neutrophil numbers in the periphery. This could be one mechanism through which adjuvant therapy works to suppress cancer recurrence11. Neutrophils are also of interest as a potential drug delivery vehicle for tumor targeted therapies to maximize efficacy and minimize toxicity12. This is most relevant within the scope of neuro-oncology treatment since neutrophils are capable of crossing the blood-brain barrier, which significantly limits the anti-tumor efficacy of conventional chemotherapeutics13.Myeloid-derived suppressor cells (MDSCs)
MDSCs result from the disruption of normal hematopoiesis and the resulting accumulation of immature myeloid cells. They are immune suppressive cells that promote cancer growth and inhibit anti-tumor immunity (i.e., MDSCs play a critical role in tumor evasion), including the inhibition of T, B, and NK cell tumoricidal activity. Growth factors produced by tumors both increase formation of MDSCs in bone marrow and recruit them to the tumor. MDSC research is focused on reducing production of these cells, infiltration of MDSCs into tumors, and interrupting their immunosuppressive functions5,14.Preclinical modeling of human myeloid cell immunotherapy
HIS mice have been used extensively in immuno-oncology research, with much early work focusing on T cell biology. Development of host strains with superior engraftment and differentiation of human myeloid cells now enables studies to look beyond T cells into the diverse human immune cell landscape. The two most commonly used host strains are the NOG-EXL and NSG-SGM3. Although these strains are both capable of reconstituting human myeloid cells, they have different phenotypic characteristics and utility. For example, there are differences in the reconstitution and kinetics of human cells, human cytokine expression profile, and their lifespan. All of these must be taken into consideration during study planning. Read the Related Taconic Biosciences Insight which compares host strains for myeloid cell humanization:
Read the Related Taconic Biosciences Insight which compares host strains for myeloid cell humanization:The field has made remarkable strides in understanding the varied roles of different immune cells in cancer, including the immune factors which may contribute to immunotherapy non-response in some patients. The complex interactions between these immune cells may require combination therapy approaches to simultaneously target multiple immune pathways and cell types for optimum efficacy. Access to appropriate preclinical models which can enable study of interactions between human immune cell subsets is critical to advancing these new therapeutic strategies.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)



