Dr. Amy Wesa, Director of Immuno-Oncology Research at Champions Oncology, recently presented a Taconic Biosciences'
webinar to share her insights on leveraging murine systems toward establishing models that epitomize patient populations and enable predictive outcomes. This presentation described how Champions Oncology is complementing PDX models with incorporated human immune cells to drive the next generation of patient-derived models.
Following her presentation, Dr. Wesa addressed numerous questions from audience participants. We present the full webinar Q&A here.
Q: Do you have any recommendations for tumor models that would allow simultaneous T cell and NK cell immune responses?
Dr. Amy Wesa: That's a challenging question. We are doing development work towards that end, but those cell types use some of the same cytokines so there can be competition between them. It's definitely an area of interest.
Q: Can you comment on the percentage of NK cells that engraft in hIL-15 NOG and how that compares to what is seen in a healthy human?
AW: In these adoptive transfer models, the cells that are present in
hIL-15 NOG mice are predominantly NK cells, so higher by percentage than you would see in circulation in a human. In a human, NK cells range from 3-12% of the total cells in circulation.
Q: For the different types of humanized models described, do you need irradiation before engraftment? Under what circumstances would you recommend irradiation?
AW: In the context of hCD34+ engraftment, we do irradiate. In the context of NK cells, we have done engraftment both with and without irradiation. There might be more wiggle room in that space. In the context of human peripheral blood mononuclear cells (PBMCs), if you're interested in graft vs. host disease (GvHD), I recommend that you irradiate because it promotes more rapid GvHD. For immuno-oncology studies, we do not irradiate prior to implanting PBMCs.
Q: Do you have experience using your acute myeloid leukemia (AML) patient-derived xenograft (PDX) models with immuno-oncology agents?
AW: This is an area of active investigation. Because the engraftment is primary AML, there are typically very low levels of T cells as they are depleted in advance. We are working to develop methods such as
ex vivo co-culture (we will launch this platform within next couple of months).
Q: Please comment on the AML PDX variability and how this variability can be addressed when planning a study.
AW: In certain models we know that there will be a range of levels of engraftment. We approach that by engrafting an overage relative to the number of mice that will go on study. We have a good idea of the level of variability for a certain model. The overage allows us to randomize to ensure that we have tight groups, with some mice going on study and some not going on study.
Q: Have you evaluated PDX with different MHC I expression levels in the hIL-15 NOG NK cell-engrafted model? For example, are you seeing differences in basal NK killing depending on whether or not the tumors are expressing MHC class I?
AW: Natural NK cell killing occurs in the absence of MHC class I on the surface of cells. We have not looked at that yet, but I think it would be interesting to address it.
Q: Are you able to engraft NK cells or other immune cells that have been genetically modified first by a third party and shipped to you?
AW: We have a lot of experience using cell therapy products, particularly in the context of CAR-T cells into our cell line xenografts or PDX. The data here showing primary specimens implanted were
ex vivo which should lend confidence that this is something we're experienced in.
Q: Can you comment on a typical quantity of AML cells you engraft per mouse or does it vary across the different AML models?
AW: There are different approaches. We tend to use a standard dose across most of our models. You can vary the dose and that may affect the kinetics of the engraftment.
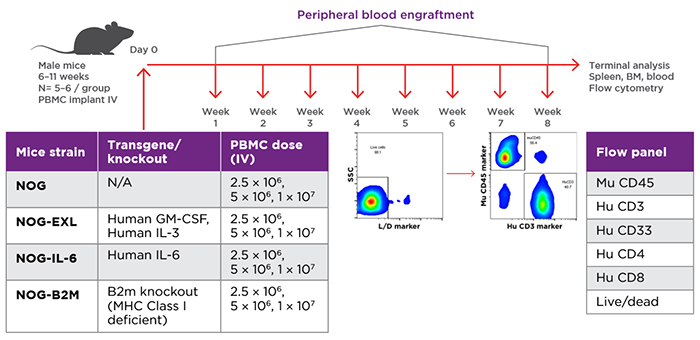
Study design for evaluation of PBMC engraftment in different NOG Portfolio strains. Source: Verma et al. 2019.
Q: Regarding use of expanded and fresh NK cells engrafted into hIL-15 NOG, do you already have banked NK cell inventory and can the cells be screened for particular properties prior to study?
AW: We are in the process of establishing a bank and may be able to select cells based on customer requirements. The other alternative is that customers can provide their own source of NK cells that have the desired characteristic, for example some particular molecular marker which may not be in our bank.
Q: You presented a number of different humanized immune system mouse models for immuno-oncology. How do you select the appropriate model system? What is your guidance for the number of donors to use in a humanized immune system mouse study?
AW: We work directly with the end user to identify the most appropriate model system. There are a number of factors to consider. For example, what are the requirements related to the tumor, for example a particular molecular marker on a PDX? What is the particular disease category? On the humanized side, what is the target cell of interest? Among the models, there are some that have differences in terms of kinetics. For example, the hCD34+ engrafted NOG model takes longer to develop compared to the PBMC-engrafted NOG model. In some cases, a GEM model may be preferable depending on what you are targeting. Regarding the number of donors, we recommend at least three donors. On average, 1/3 of donors are responsive, so we would recommend using three donors and including enough n number for each donor. If you have too few mice for certain donors it could potentially dilute the response you are testing.
Q: Do you have NK cell or other (tumor-infiltrating lymphocyte) TIL information available for humanized mice studies?
AW: We have a small cohort of models where we may have information on TILs with particular PDX. In some cases it might be important to do a pilot study looking at a particular PDX and immune cell donors. In the context of NK cell engraftment, this is something we are currently looking at.
 Watch the Taconic Biosciences' Webinar:
Watch the Taconic Biosciences' Webinar:







.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)
.jpg)


.jpg)



.jpg)




.jpg)

.jpg)
.jpg)




