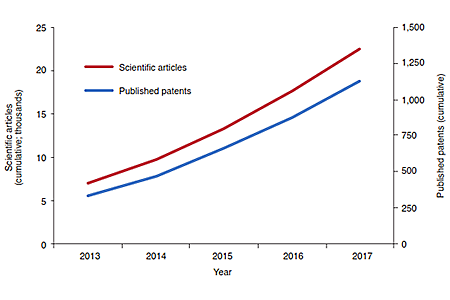
Figure 1: Over the past five years, the scientific output in the microbiome
field and the number of microbiome-related published patents has been
steadily rising. Sources: PubMed (search terms "human microbiome" and
"human microbiota"); Fankhauser et al.; University of Chicago Technology
Commercialization; CBInsights; CrunchBase; GlobalData; the companies.
The Translating Microbiome Futures publication provides the key takeaway messages provided by sixteen leaders in the microbiome field. Alexander Maue, PhD, Director of Microbiome Products and Services at Taconic was one of those interviewed. During his interview Dr. Maue was asked, "Given the limitations of current preclinical models, what kinds of advances do you anticipate are needed to facilitate microbiome product development?"
He responded, "One way of addressing some of the present limitations is to use animals with humanized immune systems that have been associated with human microbiota." He went on to say, "An additional strategy would be to perform experiments on offspring of transplanted parental animals that would have co-developed their immune systems with the transplanted microbiome — the diversity of the transplanted microbiome has been shown to be conserved in the F1 generation."
This publication is timely as we enter 2019 and look to gain additional knowledge of how the microbiome is associated with health and disease. During 2019, more therapies will head towards the clinic and still others will seek regulatory approval. The authors have provided a perspective on the challenges that face researchers and regulators over the coming years, as well as some insight on how we may overcome some of those challenges.






.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)
.jpg)


.jpg)



.jpg)




.jpg)

.jpg)
.jpg)




