Addressing cross species differences in cytokine signaling through generation of novel transgenic mouse models to better support human immune system engraftment
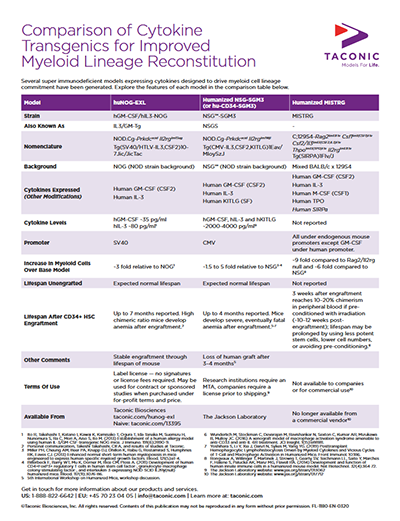
Several groups reported generation of new models via novel human cytokine expression methods onto existing super immune deficient host mice. By presenting a series of random pronuclear transgenics and targeted knock-ins, various researchers sought to directly test if human variants of key cytokines could rescue sub-lineage commitment after engraftment with human hematopoietic stem cells (HSC). With multiple groups addressing the same restrictions to NK cell, dendritic cell, and other myeloid lineages, some consensus to the limiting biology was reached by independent investigations at the Central Institute for Experimental Animals (CIEA), Regeneron Pharmaceuticals, and Yale University. Two different human IL-6 transgenic models were described by the Flavell Lab at Yale and the Central Institute for Experimental Animals (CIEA) in Japan. Both demonstrated that endogenous (via knock-in) or exogenous (via random transgenesis) production of human IL-6 impacted the frequency and functional differentiation of human monocytes. Human IL-15-expressing models were reported by Regeneron and CIEA, with both groups observing rescued NK cell differentiation in the presence of human IL-15.- CIEA with a NOG mouse expressing human GM-CSF and human IL-3 [NOG-EXL (hGM-CSF/hIL-3 NOG)]
- The Jackson Laboratory with an NSG mouse expressing human IL-3, GM-CSF and Kit ligand [NSG-SGM3]
- The Flavell Lab with a mixed background BALB/c x 129S4 Rag2/Ilrg double knockout expressing human M-CSF, GM-CSF, IL-3, TPO and a human SIRPα allele [MISTRG]
The problem of secondary lymphoid tissue development in humanized mice
There is a well-established developmental requirement for gamma-chain dependent cytokine signaling during organogenesis in order to fully form the peripheral lymphatic system. However, the most common immune deficient host mice contain germline disruptions of the IL2 receptor gamma chain, resulting in disorganized, dysfunctional or often absent lymph nodes. NOG, NSG and BRG mice, the most receptive immune deficient host models for humanization, all contain targeted mutations of the IL2 receptor gamma chain, resulting in poor establishment of the proper architecture necessary for productive germinal center formation and consequent robust adaptive immune responses upon humanization. In the most remarkable talk of the meeting, Takeshi Takahashi of CIEA reported the generation of a BAC transgenic NOG mouse that expressed a functional IL2 receptor gamma chain (IL2rg) under the control of the mouse RORγt promotor and regulatory sequence. Because the RORγt is highly expressed in lymphoid tissue inducer cells, Takahashi hypothesized that selective gamma chain expression in this specific cell type would rescue lymph node formation while retaining the immune deficiency critical for accepting human HSC engraftment. Indeed, his transgenic model developed sustained lymph nodes and may provide an improved environment for B cell maturation and Ig class switching.In another study, Yan Li of the DiSanto Lab at Institut Pasteur described a BRGS mouse model (BALB/c Rag2/Il2rg knockout with transgenic expression of the NOD Sirpα allele) that additionally expresses thymic stromal lymphopoietin (TSLP), a key cytokine for CD4+ T cell homeostasis. These showed improved lymph node development and enhanced Ig class switching. Li also described a system for exogenous administration of TSLP in utero that could be employed to circumvent the requirement for a further genetically modified strain. Despite the improvements in lymph node development, neither group reported development of Peyer's Patches. Inconsistent development of Peyer's Patches was reported by Cagan Gurer of Regeneron in an SRG (Rag2/Il2rg knockout with a human SIRPα allele) mouse with human IL-15 knocked into the mouse Il-15 gene.
Modeling human disease and utility of humanized models in drug discovery
The program included sessions on both infectious and malignant disease. Florian Klein of the University Hospital of Cologne presented a plenary lecture discussing advances in HIV-1 research using humanized mice. One aspect highlighted in his talk was identification of resistance mechanisms such as escape mutations in HIV envelope protein after monotherapy. His group used human immune system engrafted mice to study broadly neutralizing antibody therapy against HIV, in a project that has advanced to human clinical trials.New cytokine transgenic models also yielded improvements in human disease modeling. Ryoji Ito of CIEA discussed advances in asthma modeling in transgenic NOG mice expressing human GM-CSF, IL-3 and IL-5. Upon administration of human IL-33, Ito detected immune responses consistent with asthmatic airway inflammation including goblet cell hyperplasia, periostin secretion in BALF and eosinophilic inflammation.
Kathie Beland of the CHU Sainte-Justine Research Centre described a new model of Rasmussen's encephalitis induced via engraftment of PBMCs from patients with this seizure disorder. Engraftment with the patient-derived cells caused seizures in the mice. This model could further investigations into the etiology of Rasmussen's, which has been suspected to have an autoimmune component.
One of the select presenters representing large pharma drug discovery was Johannes Sam of Roche. Sam discussed an immuno-oncology application: The use of NOG mice engrafted with human CD34+ HSCs for validation of T cell bispecific antibodies. His experimental model included the generation of large cohorts of humanized mice using cord blood-derived cells. 14-16 weeks after HSC engraftment, mice were randomized and engrafted with a human tumor cell line. After establishment of tumor growth, treatment with T cell bispecific antibodies or various other treatments was initiated. To determine if the bispecific antibody was capable of activating a T cell-mediated response against the tumor burden, both tumor regression and T cell infiltration were measured. Sam indicated the successful resolution of multiple tumor cell lines suggests this model has great utility for drug discovery, but added a note of caution because complete understanding of the model itself is necessary to understand the potentially confounding instances of adverse toxicity unrelated to the drug administration.
Limitations of current models
During the Q&A periods and a roundtable discussion, attendees engaged in frank discussion about the challenges and limitations associated with some of the commercially available models. The onset of anemia was described as a limitation for many of the models with improved myeloid reconstitution. For example, the MISTRG mouse has a narrow window of ~3 weeks after sufficient engraftment levels (10-20% chimerism) are reached before severe anemia sets in. The hGM-CSF/hIL-3 NOG and NSG-SGM3 strains have a longer useable window post-engraftment. Several hypotheses to explain the onset of anemia were suggested, including:- Non-T cell mediated graft attack of host cells due to engulfment of mouse erythrocytes by higher frequencies of human macrophages.
- Disruption of mouse hematopoiesis by human hematopoietic stem cells (HSCs) pushing mouse HSCs out of the bone marrow niche and/or CSF mobilizing stem cells.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)