 There is an urgent need to find mouse models to support the development of SARS-CoV-2 vaccines and therapeutics and combat the COVID-19 pandemic. A recent study from Peking Union Medical College has demonstrated the promising application of humanized angiotensin converting enzyme 2 (ACE2) transgenic mice for SARS-CoV-2 research1.
There is an urgent need to find mouse models to support the development of SARS-CoV-2 vaccines and therapeutics and combat the COVID-19 pandemic. A recent study from Peking Union Medical College has demonstrated the promising application of humanized angiotensin converting enzyme 2 (ACE2) transgenic mice for SARS-CoV-2 research1. Mouse models are widely used to develop and test the safety and efficacy of vaccines and antiviral drugs, as well as provide important insights into disease pathogenesis. Like the related SARS-CoV, SARS-CoV-2 utilizes human ACE2 as a receptor on human target cells for viral entry. These viruses have a much lower affinity for mouse ACE2, which means that normal mice cannot be used for viral infection studies using clinical isolates, and special mouse-adapted viral strains are required for in vivo infection models. Although mouse-adapted SARS-CoV-2 has reportedly been developed, alternative models, such as primates or specialized genetically engineered mice, are still sorely needed in the fight against COVID-19.
Mice expressing human ACE2 are needed for COVID-19 infection models
Transgenic mice expressing the human version of ACE2 (hACE2) allow for effective viral infection with clinical (non-mouse-adapted) SARS-CoV isolates2-6. These models also demonstrate a range of SARS-CoV-induced pathology, including pneumonia. A similar strategy was also effective for MERS-CoV using human knock-in mice for its receptor, DPP47.This new study by Linlin Bao and colleagues showed that this approach also applies to SARS-CoV-21. They report that hACE2 transgenic mice can support effective SARS-CoV-2 infection with weight loss and mild to moderate pneumonia with interstitial hyperplasia. In contrast, wild type non-transgenic controls were resistant to SARS-CoV-2 infection. In infected hACE2 transgenic mice, viable virus was present in lung tissue, and viral RNA was detected in multiple organs, most notably lung, spleen and liver. However, other than mild body weight loss, there were no other reported clinical signs of disease during the study period (3-5 days post-infection).
These results are extremely encouraging and suggest that any of the hACE2 transgenic models that were originally developed for SARS-CoV may be relevant for SARS-CoV-2. At least nine different hACE2 transgenic lines were developed in 2007 by groups at the University of Iowa3,5, the University of Texas Medical Branch4,6, and Peking Union Medical College/Peking University2. Yet another line was generated in 2016 at the University of North Carolina for use studying a SARS-like virus called WIV18. Unfortunately, many of these have been sitting unused as cryopreserved sperm or embryos given a lack of funding for coronavirus research. Peking Union Medical College has a live line, as reported in the recent Nature report, and The Jackson Laboratory is working to cryorecover a second from Stanley Perlman at the University of Iowa. Recently, Taconic Biosciences announced that it has obtained a number of hACE2 lines from Dr. Chien-Te K. Tseng at the University of Texas Medical Branch and is using advanced in vitro fertilization techniques to rapidly scale up production in preparation for global distribution.
March 2021 update: The hACE2 AC70 and AC22 mice are both now available for distribution
Distinct hACE2 transgenic lines will have functional differences
These hACE2 transgenic mice will be invaluable in the testing and development of COVID-19 vaccines, and therapeutic or prophylactic antiviral therapies. But why the rush to make so many different lines available when there is already one showing promise? As anyone familiar with transgenic mouse lines knows, the devil is certainly in the details. These models all rely on a process known as random transgenesis, in which a gene (like hACE2) is randomly inserted into the genome and its expression is controlled both by a promoter as well as local (cis-acting) and distant (trans-acting) modifiers in the mouse genome. Furthermore, this insertion may happen at multiple sites, and may interfere with normal mouse biology, such as reproductive performance. These factors make each transgenic mouse line unique and will influence how these lines respond to SARS-CoV-2 infection as well as the stability of the different transgenes and the speed at which we are able to quickly produce sufficient numbers to meet research needs.The various hACE2 transgenic lines show different responses to SARS-CoV infection, and we can reasonably assume that the same will hold true for SARS-CoV-2. These differences are summarized in Table 1. On one hand, there are the lethality-sensitive strains, which quickly succumb to SARS-CoV infection and die within 3-7 days post-infection. Then there are the lethality-resistant strains, which develop disease but eventually recover. Each of these strains initially develop lung pathology with active viral replication in the lung and varying degrees of local inflammation. However, particularly in the lethality-sensitive strains, the infection progresses to extensive infection in the brain and neurological damage. Original reports indicate that the cause of death in these strains is likely aspiration, caused by neurological damage, as opposed to pneumonia or respiratory failure. Although SARS-CoV has been identified in brain sections from SARS patients, the clinical significance of this is unclear. In contrast, the lethality-resistant strains may have a much lower level of brain infection, which may be associated with lower hACE2 expression levels.
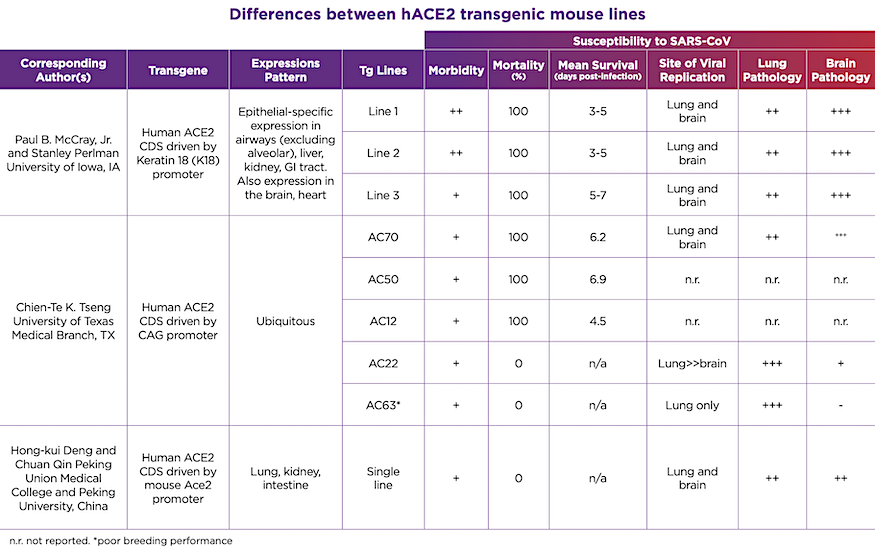
Table 1. Differences between hACE2 transgenic mouse lines
More research needed to identify most appropriate hACE2 line for particular COVID-19 research applications
It is unclear which of these transgenic lines would be the most appropriate for SARS-CoV-2 research, or whether certain lines would lend themselves to different types of studies. The Linlin Bao report used a lethality-resistant strain and, just as with SARS-CoV infection, these mice only showed mild clinical signs of disease with mild to moderate lung pathology in response to SARS-CoV-2 infection. Would a lethality-sensitive strain be better to understand the pathology and test treatments for critically ill patients? How would extensive brain infection in some of these models interfere with efforts to test antibody neutralization therapeutics, given the difficulties in delivering large molecules to the CNS? Which model would be best to test the efficacy and safety of various vaccination approaches?There are many questions in this time of uncertainty, but rational science-based investigation offers the promise to develop effective strategies to address this pandemic and prevent future outbreaks. Preclinical studies in animals will be critical in this, just as they have been for countless other pathogens and diseases. These hACE2 transgenic mice, in particular, will be invaluable to quickly and efficiently test the numerous therapeutic strategies currently being proposed to help end the COVID-19 pandemic.
Stay safe and wash your hands.
- Mouse-adapted SARS-CoV-2 strains unlock broader mouse modeling of COVID-19
- An Overview of Mouse Models for COVID-19
- Safety Testing Critical for COVID-19 Therapies and Vaccines
- Knockout Mice for the Study of Coronavirus Infections
- Aged Inbred Mice May Advance Research Into Age-Related Mortality in Coronavirus Infections















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)




