Although the exact details are not understood, the immune system is involved in MS pathogenesis. Like MS, EAE is characterized by infiltration of immune cells into the central nervous system (CNS) and demyelination. The two most widely-used methods to induce EAE are:
- Active induction by immunization.
- Passive induction through adoptive transfer.
EAE by Active Immunization
Active immunization models are the most popular mouse models of multiple sclerosis. In the active immunization method, the mouse is immunized using neuro antigens, such as myelin antigens or spinal cord homogenate, which causes development of activated myelin-specific T cells, trafficking of immune cells into the CNS, and direct damage and inflammation in the CNS.General Benefits and Considerations
- Quick, relatively easy, and inexpensive.
- Active immunization in Black 6 mice is suitable for compound profiling.
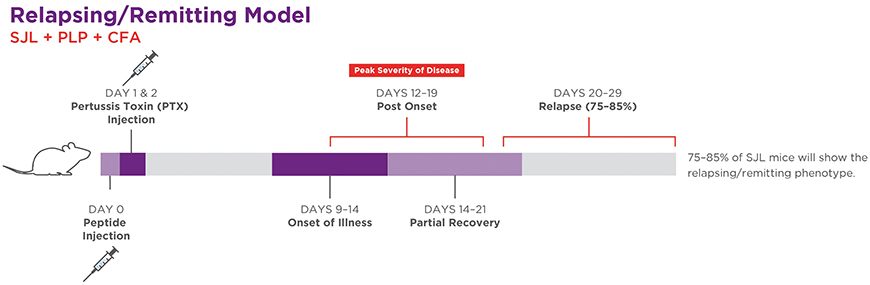
- Active immunization in SJL mice induces a relapsing/remitting disease phenotype.
- Antigens for EAE induction include myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), proteolipid protein (PLP), and spinal cord homogenate.
- Most protocols call for the use of pertussis toxin (PTX), which increases both the incidence and severity of disease.
- Disease presents as ascending paralysis and the typical readout is clinical score. Histology may be used to visualize inflammation in the CNS.
- The Gfap-luc mouse model offers an additional readout: in vivo imaging of bioluminescence as a marker of neuroinflammation. (Note that the Gfap-luc model on the B6 albino x FVB hybrid background must be used in order to maintain susceptibility to EAE.)
Basic Protocol

Adoptive Transfer EAE Mouse Models
In the adoptive transfer model of EAE, one set of mice is directly immunized to generate myelin-specific T cells which are then cultured and transferred into donor mice.General Benefits and Considerations
- Rapid onset and increased disease severity.
- The adoptive transfer model permits separation of the effector phase from induction.
- Congenic pairs of donors and recipients can be used to study cell trafficking. Using B6.SJL-Ptprca mice as donors and C57BL/6 mice as recipients, donor T cells can be tracked in vivo in the recipient mice.
- Study differentiated Th1 or Th17 populations; in vitro culture conditions can be varied to alter the dominant cell type in the transferred cells.


Strain Selection for EAE Mouse Models
Black 6 on the J and NTac background exhibit comparable EAE development and clinical scores, with C57BL/6NTac having slightly higher clinical scores in the initial onset and the J having slightly higher relapse scores.
Age and sex matched Black 6 mice used in active immunization study using MOG and CFA. Protocol available from Hooke Labs.

C57BL/6 Mice from different Taconic barriers with the same health status. There is consistency in data due to harmonization. Data collected by Hooke Labs.
- Take advantage of our Smart Select Program. Smart Select is a complimentary animal model trial program allowing you to trial up to 20 animals in your facility at no cost to your organization. Learn more today.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)




.jpg)

.jpg)




