Humanized immune system mice are valuable tools for a wide variety of research including studies in immuno-oncology, autoimmune, and infectious disease. But understanding the utility of such models has been hampered somewhat by the wide variety of humanization protocols in use as well as donor-specific effects.
The Details Matter When It Comes to Humanization
The process to generate a humanized immune system mouse is complex. Many different host strains are available, and while some of the differences in humanization outcome by strain are understood, others remain to be elucidated. Age and sex of mice may also influence the outcome. Optimal engraftment of human hematopoietic stem cells (HSC) requires a myeloablation step, and this can be accomplished via several different methods. HSC source, cell dose, and injection method may further influence the outcome. All of these variables mean it can be challenging to draw firm conclusions such as "humanized mice can do X" or "humanized models can't do Y", as the results of a particular study may only apply to a particular combination of strain, engraftment method, and cell source or donor rather than more broadly to "humanized mice".
True head-to-head comparisons of humanized models are rare, but a recent paper in Frontiers in Immunology does just that. This well-designed study by Maser et al. allows for clear conclusions regarding several popular humanization host strains: NOG, NOG-EXL, and NSG-SGM3.
Comparing Commonly Used Host Strains
Scientists at Roche and FAU Erlangen performed a head-to-head comparison of two commonly used humanization host strains designed to support human myeloid cells, the NOG-EXL and the NSG-SGM3. These were also compared to the base NOG model. All strains were humanized using the same engraftment protocol and even the same human cell donors so as to be able to draw strong conclusions absent variables introduced by technique and donor (Figure 1).
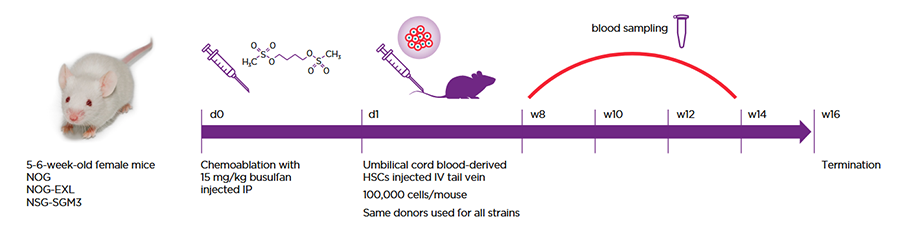
Figure 1: All strains were humanized via the same protocol and with the same HSC donors.
Some of the results of the head-to-head comparison of the three strains confirmed past reports. Unsurprisingly, NOG-EXL and NSG-SGM3 supported higher overall levels of human cell engraftment compared to the base NOG strain. Myeloid-enhanced strains are known to have a decreased lifespan relative to base strains like NOG or NSG, with this previously reported as a more severe problem in NSG-SGM3. This is the first paper to show, using a true head-to-head comparison that humanized NOG-EXL have a survival advantage over humanized NSG-SGM3 (Figure 2).
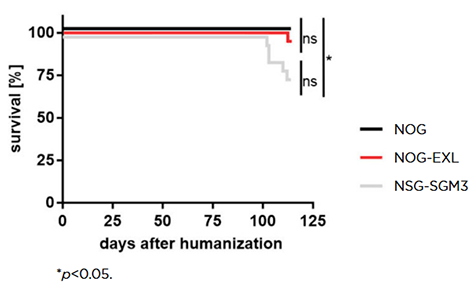
Figure 2: Humanized NOG-EXL mice exhibit improved overall survival compared to humanized NSG-SGM3 mice. Copyright © 2020 Maser, Hoves, Bayer, Heidkamp, Nimmerjahn, Eckmann and Ries. Licensed under CC BY 4.0 (www.creativecommons.org/licenses/by/4.0/).
This conclusion of significant post-humanization lifespan differences between NSG-SGM3 and NOG-EXL has been validated by other groups, for example by Willis et al.
Using consistent donors and technique, Maser et al. found that while both the NOG-EXL and NSG-SGM3 supported higher levels of human myeloid cell differentiation and improved overall human cell reconstitution compared to NOG, the humanized NOG-EXL represented most closely the immune cell composition found in humans. Further, humanized NOG-EXL maintained frequencies of human myeloid and NK cells whereas those populations declined in frequency in humanized NSG-SGM3 mice over time. Differences in myeloid cell composition between humanized NOG-EXL and NSG-SGM3 were seen, including higher levels of granulocytes in bone marrow and spleen of NSG-SGM3.
Human Dendritic Cells in HIS Mice
Maser et al. looked in more detail at dendritic cells in these humanized models. All three major classes of dendritic cells, including classical CD1c+ DCs (cDC2), cross-presenting CD141+ DCs (cDC1) and plasmacytoid CD303+ DCs (pDC), were found in humanized NOG, NOG-EXL and NSG-SGM3. Humanized NOG-EXL mice had the highest overall level of DCs, and pDCs from these mice were functional. pDCs are of interest as an immunotherapy target, both to directly kill tumor cells as well as for their role in regulating Treg suppressive functions.
Comparing humanized NOG and NOG-EXL mice in tumor engraftment experiments, Maser et al. discovered that composition of tumor-infiltrated human immune cells was affected more by tumor type than by mouse strain. Two out of three tumors tested contained human pDCs as the predominant human DC type found in the tumors, which may be due to the specific profile of tumor-generated chemokines and cytokines.
Impact of this Work
This work is significant on two fronts. First, the true head-to-head comparison in humanization of three host strains is an important contribution to the humanized mice literature. Second, it presents a model system for the further evaluation of pDC-targeting as an immuno-oncology therapeutic strategy. Enrichment of this rare human cell type in humanized NOG-EXL provides a ready system to study the normal function of pDCs and test approaches to manipulate pDCs for therapeutic aims.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)





