While checkpoint inhibitors have been a great leap forward in the treatment of certain cancer types, only a limited portion of patients respond satisfactory to the therapy. Many researchers have come to suspect the gut microbiome plays an important role in modulating this response.
Both studies tracked the responses of cancer patients to immune checkpoint inhibitors, a type of immunotherapy that prevent cancer cells from switching off T cells' anti-cancer activity, and looked for possible involvement of the microbiome in patient responses.
In the Science papers, it was shown that responders and non-responders have different compositional and functional features of their gut microbiomes. It appears beneficial for the efficacy of checkpoint inhibitor therapy to harbor high levels of Akkermansia munciniphila, Faecalibacterium and other specific commensals in the gut.
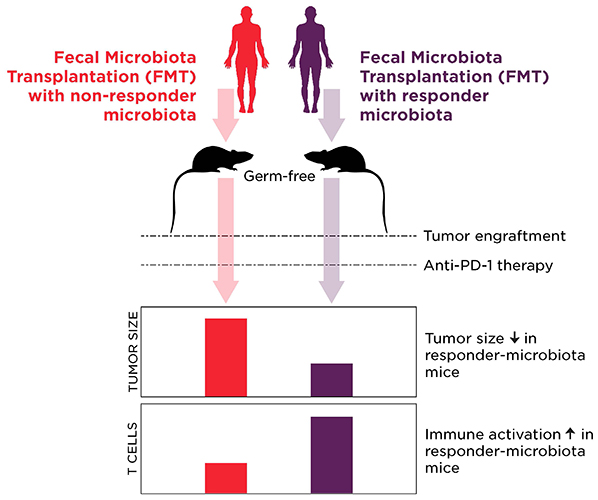
The authors used germ-free mice in both studies to demonstrate a potential causal role of the microbiome by transplanting feces from the human patients into the mice. The results were unequivocal: in both cases, mice receiving gut microbiota from non-responders did poorer on immune checkpoint inhibitor therapy than mice colonized with feces from responders.
Immunological profiling of the patients and the mice suggested that the favorable microbiome of responders enhanced systemic and anti-tumor immune response, whereas the microbiota of non-responders was causing the host to have impaired anti-tumor immune responses.
These intriguing studies not only demonstrate a strong correlation between microbiome composition and efficacy, but suggest new options to exploit the microbiome to improve the efficacy of cancer therapy:
- If possible, avoiding antibiotics prior to and while taking checkpoint inhibitors may improve the response to the therapy.
- The microbiome may be a predictor of response to therapy, enabling targeted modulation of the microbiome from an early time point.
- Clinical trials testing the benefit of modulating the microbiome of cancer patients receiving checkpoint inhibitors are already underway. Seres Therapeutics recently announced the plans for testing their preclinical live bacteria candidate SER-401 in combination with anti-PD-1 therapy.
Summary of key findings of the two papers
| Patient groups | Key gut microbiota observations | Key clinical observations | Key results from patient-to-mouse Fecal Microbiota Transplantation (FMT) | |
| Routy et al. | Non-small cell lung carcinoma (n=140) Renal cell carcinoma (n=67) Urothelial carcinoma (n=42) 28% of patients had taken antibiotics within 2 months prior to start of therapy Checkpoint inhibitor therapy: Anti-PD-1/PD-L1 | Higher abundance of Firmicutes, Akkermansia muciniphila and other specific taxa in responders to therapy | Progression-free survival and overall survival shorter in antibiotic-treated patients | No difference in tumor growth between untreated mice with microbiota from responders or non-responders Mice with responder-microbiota had improved response to therapy Mice with responder-microbiota had more CXCR3+CD4+ T cells in tumor microenvironment |
| Gopalakrishnan et al. | Metastatic melanoma (n=112) Checkpoint inhibitor therapy: Anti-PD-1 | Higher alpha diversity in responders to therapy Higher abundance of Faecalibacterium, Ruminococcaceae and other Clostridiales in responders Higher abundance of Bacteroidales in non-responders | Progression-free survival shorter in patients with low Faecalibacterium and high Bacteroidales abundance Positive correlation between CD8+ T cells in tumor microenvironment and abundance of Faecalibacterium, Ruminococcaceae and Clostridiales in the gut | Mice with responder-microbiota had reduced tumor growth before therapy Mice with responder-microbiota had improved response to therapy Mice with responder-microbiota had more CD8+ T cells in tumor microenvironment |















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)




