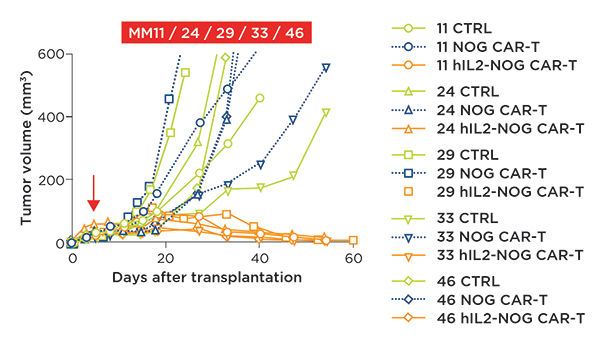
Figure 1: HER2 CAR T cell efficacy in vivo is only observed in the hIL-2 transgenic NOG. Combined
data of tumor growth curves of 5 different PDX models of cutaneous melanoma (MM11, 24, 29, 33, 46)
in NOG or hIL-2 NOG. All 5 patient PDXs were responsive to HER2 CAR T cell therapy with a
significant reduction in tumor volume, however, this efficacy was only present in the
hIL-2 NOG model.
T-Cell Therapy Challenges
To date, there are no CAR-T therapies approved for solid tumor treatment1,3. These might be due to limited access, the heterogeneity of the tumor, T cell exhaustion, or a microenvironment that lends itself to immune evasion, indicative in the solid tumor1,3,4.In general, tumors escape T-cell activation by:
- Poor immunogenicity
- Their cells are not considered phenotypically foreign by the host
- Tumor-associated immunosuppression3
Checkpoint Inhibitors
Advances in understanding the immune system pathways underlying T cell function show an intricate balance of activation and inhibition mediated via receptors3. This functional dichotomy of T cell receptors enables a balance between an immune response and tolerance3. Immune checkpoint inhibitors work to remove this inhibition (or brake) of the system, combined with autologous adoptive cell therapy (ACT) with CAR-T or tumor infiltrating lymphocytes (TILs), have highlighted the potent clinical efficacy of engaging the immune system to target tumor cells1-6.In particular, immunotherapies (specifically checkpoint inhibitors) have significantly advanced the treatment of metastatic malignant melanoma1,2. However, these therapies require an immune reaction via tumor-specific antigens (neoantigens) and some forms of melanoma show little, if any (e.g. uveal melanoma)1.
Evaluating CAR-T Cell Therapies in Humanized Mice
Recently, researchers attempted to address this lack of immunogenicity by using a CAR-T cell engineered with a CD3 signaling domain fused to a costimulatory lymphocyte receptor (CD28 and/or 4-1BB) and an external receptor targeting a protein found on the surface of tumor cells (the oncogene human epidermal growth factor receptor 2, HER2 or ERBB2)1,6. These CAR-T cells were generated from peripheral blood mononuclear cells (PBMCs) of five different donors and were evaluated both in vitro and in vivo for their cytotoxicity against HER2-expressing (the only oncogene present in these tumor cells from established CAR-T targets) cutaneous and uveal melanoma1.The HER2 CAR-T cells were evaluated for their target-dependent cytotoxicity by exposure to cultured "low" (SK MEL-1) and "high" (HS695) HER2 expressing melanoma commercial cell lines. The efficacy of the HER2 CAR-T was higher in the "high" expressing cell line and further evaluation of on-target activity was demonstrated with the use of a HER2 KO via CRISPR/Cas9. CAR-T cytotoxicity was targeted to HER2 and rescue of the tumor cells was shown with HER2 deletion in the cell line.
Addressing Treatment-Resistant Melanomas
With the on-target efficacy of CAR-T demonstrated in HER2 expressing melanoma lines, the authors sought to address whether CAR-T therapy could be an option for patients that show resistance to ACT of TILs and/or checkpoint inhibition (PD-1).The authors developed a cell line from the subcutaneous melanoma tumors of patients who were either resistant (MM46, MM11-NR) or regressed (MM33) to their own TILs. Treatment with HER2 CAR-T resulted in tumor cell death irrespective of their response to their own TILs. HER2 deletion was able to rescue the cells from death showing a HER2-directed cytotoxicity. Evaluation of HER2 CAR-T efficacy was also demonstrated on two commercial uveal melanoma cell lines (92-1 and MEL202). The in vitro work showed the on-target efficacy of the HER2 CAR-T therapy in both types of melanoma and in PDXs resistant to ACT TIL therapy.
For the in vivo evaluation, the authors selected PDX melanoma cell lines that were sensitive to CAR-T cells grown in either NOG or hIL-2 NOG mice, as a previous study showed that ACT TILs only show efficacy in the hIL-2 NOG model due to the required chronic presence of IL-2. After successful PDX transplantation, ACT of TILs or CAR-T therapy was given to hIL-2 NOGs, while CAR-T therapy or a control was administered to NOG mice. In all five PDXs, NOG plus CAR-T therapy did not show any efficacy on tumor growth, whereas hIL-2 NOG showed significant CAR-T mediated anti-tumor efficacy (see figure, below).
This response occurred irrespective of whether the PDX was resistant to TILs or not. Their previous study, the authors showed that the PDXs that are resistant to TILs in the hIL-2 NOG corresponded to the patient's clinical ACT response, demonstrating the predictive validity of the model2. To further solidify the robustness of the HER2 directed CAR-T therapy, a PDX (M33F8) was transplanted in triplicate within the hIL-2 NOG and then subjected to CAR-T, with significant anti-tumor effects compared to controls seen in all three1.
Next, the authors evaluated the CAR-T therapy in both a commercial cell line (92-1) and PDX (UM121213B) of uveal melanoma, a cancer that is resistant to immunotherapy. In both instances, efficacy was seen1.
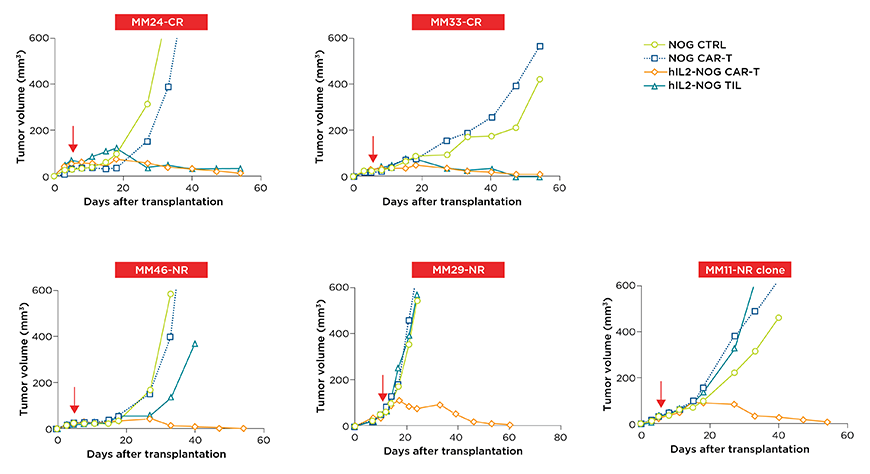
Figure 2: Patient screened cutaneous melanoma PDXs respond to HER2-CAR T therapy in the hIL-2 NOG regardless of whether or not they were clinically resistant to adoptive cell therapy (ACT) of tumor infiltrating T lymphocytes (TILs). In the hIL-2 NOG, CAR T cells showed a significant reduction in tumor volume and corresponding cell death, irrespective of whether these cells could respond to their own TILs (top row TIL sensitive, bottom row TIL resistant PDXs).
Summary
In conclusion, the authors were able to demonstrate in vivo efficacy for HER2 CAR-T therapy in solid melanoma tumors irrespective of previous immunotherapy resistance/remission1. This efficacy was only seen in the hIL-2 NOG model. The use of the hIL-2 NOG is required for the efficacy evaluation of both ACT of TILs and CAR-T therapy in solid melanoma tumors1,2.Furthermore, the potential predictive validity of the model (already demonstrated in the ACT of TILs) for CAR-T cell use in solid tumors is apparent. This positions the IL-2 NOG model as an excellent preclinical model for the efficacy of immunotherapies implicating T cell activity (checkpoint inhibitors and ACT of either TILs or CAR-T)1,2.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)




