The Problem with SPF Mice
Historically, laboratory mice have been valued for their well-defined genetics and a health profile free from mouse pathogens. Over the last thirty years, efforts have been made to improve the health of laboratory mice by removing organisms that are deemed pathogenic. That list is now approaching one hundred organisms. While there are clear benefits to removing pathogenic organisms from laboratory mice, there were unintended consequences: along the way, many beneficial or commensal organisms were also removed. Those drawbacks came to light in two 2016 publications1,2 and now, very elegantly, in a new published study.Wild Mouse Gut Microbiota
Stephan Rosshart (and colleagues) of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) recently published a study demonstrating how the "sanitized" laboratory microbiome performs very differently from microbiomes derived from wild-caught mice. They note laboratory mice are not exposed to the variety of organisms that wild mice are exposed to throughout their life.
Figure 1: Source
Their hypothesis? "[L]aboratory mice lack important symbiotic host-microbe interactions crucial for host physiology, including immunity, found in wild mice, which evolved under greater evolutionary pressure from infectious diseases and naturally occurring inflammatory stimuli."To test their hypothesis, they caught more than 800 mice from horse barns in the area surrounding the National Institutes of Health in Bethesda, MD USA. After imposing some selection criteria, they identified ninety-eight mice that were unambiguously Mus musculus domesticus and characterized their mucosal-associated and luminal bacterial microbiome.
One finding was "the gut microbiome clusters from the wild mice were separate and significantly different from the gut microbiome of C57BL/6 mice from Taconic Biosciences, Charles River Labs, and The Jackson Labs."
They next cryopreserved the wild mouse gut microbiome for bio-banking and for future engrafting into lab mice, with the goal of comparing the effects of "wild" and "commercial" microbiomes on host responses to infectious and inflammatory diseases.
Prior to using the biobanked material for fecal microbiota transfer, they tested samples for known mouse pathogens by PCR and antibody detection. They were able to identify fecal material that was free of mouse pathogens. (In other words, they caught SPF wild mice.) This is an important finding in that it allows the microbiota from these mice to be used in a laboratory mouse facility without risk of contaminating other mice in the facility.
Fecal Microbiota Transfer
In the next phase of the study, timed-pregnant, germ-free C57BL/6NTac female mice, provided by Taconic Biosciences, were gavaged "wild" ileocecal material on three consecutive days starting at day fourteen of gestation. This allowed for vertical transmission of "wild" microbiome from pregnant mothers to offspring. The newborn mice acquired their "wild" microbiome through the birthing process. Other germ-free mice were gavaged with SPF ileocecal material from lab mice to act as controls.Unweighted and weighted UniFrac analysis showed that the transferred "wild" and SPF microbiota was stable over four subsequent generations of breeding. Given the differences between the typical habitat of a wild mouse and the sterile laboratory environment, it is interesting that the "wild" microbiome remained stable.
Testing Immune Response
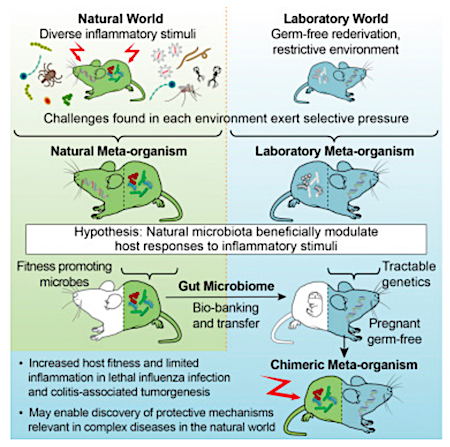
Figure 2: Source
Only 17% of the mice harboring the SPF microbiota survived the influenza challenge, compared to a 92% survival rate in mice harboring the "wild" microbiota.
Mice harboring the "wild" microbiota also did not lose weight or exhibit disease severity when compared to mice harboring the SPF microbiota. Additional data show that the mice harboring the "wild" microbiome mounted a significantly stronger immunological response than mice harboring the SPF microbiota.
To test if the "wild" microbiome would also prove advantageous in non-infectious disease, Rosshart and colleagues subjected the mice to a mutagen/inflammation-induced model of colorectal cancer. Consistent with the influenza model, mice with "wild" microbiota displayed significantly less weight loss compared to the SPF microbiota mice in this model of tumorigenesis. Additionally, the "wild" microbiota mice had a lower tumor burden and reduced inflammation.
Collectively, the authors concluded "the wild mouse gut microbiome confers traits that promote host fitness and limit inflammation in an infectious disease and in mutagen- and inflammation-induced neoplastic development".
Improving Mouse Model Microbiomes
Rosshart and colleagues have demonstrated the wild mouse bacterial gut microbiota can be viably cryopreserved and transferred to a germ-free lab mouse by FMT, with stability over several breeding generations. They also demonstrated that the wild gut microbiota promoted host fitness and limited inflammatory response when compared to laboratory mouse microbiota.In conclusion, they state "our approach should facilitate the development of animal models that better recapitulate complex natural physiological phenomena. While recent studies have pursued this goal by exposing laboratory mice to selected pathogens2 or co-housing with pathogen-infected pet store mice1, we demonstrate that host fitness promoting traits relevant in the natural world can be conferred by a natural microbiota devoid of known mouse pathogens."
Taconic was pleased to support this study through the contribution of timed-pregnant, germ-free C57BL/6NTac mice as well as Murine Pathogen Free™ (i.e. SPF) C57BL/6NTac mice. Taconic continues to lead the way in supporting microbiome research by providing germ-free standard commercial mouse strains, germ-free derivations of customer models and microbiome support services such as Fecal Microbiota Transfer. Learn more about Taconic's Microbiome Products and Services.
To read more about this research study and its impact in improving the translational value of laboratory mice, please read the NIDDK Director's Update article.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)





