Alternatives to Consider
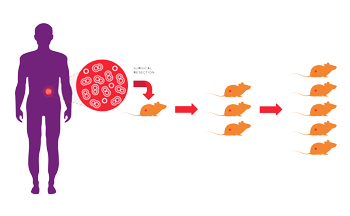
One alternative that has emerged in a powerful way is patient-derived xenografts (PDX). As noted in a commentary in Clinical Cancer Research, "Compared with xenografts from previously established cell lines, patient-derived xenografts may more faithfully recapitulate the molecular diversity, cellular heterogeneity, and histology seen in patient tumors, although other limitations of murine models remain."What are patient-derived xenografts (PDX)?
PDX are xenografts developed directly from primary human tissue. A consented patient with cancer has a surgical resection as part of his or her treatment plan. Part of the resected tissue is used to develop a xenograft. Typically the original surgical specimen is dissected into small fragments or dissociated into a single cell suspension and then implanted in a small number of immunodeficient mice. Samples that successfully grow are harvested and expanded in another round of immunodeficient hosts. This process is called passaging. To keep the PDX as close to the patient as possible, passage numbers are restricted to reduce adaptation of the tumor to the mouse host.What are the benefits of PDX?
According to a recent review article, "they are biologically stable when passaged in mice in terms of global gene-expression patterns, mutational status, metastatic potential, drug responsiveness and tumour architecture." Unlike cell lines, which have been adapted to prolonged in vitro culture, patient-derived xenografts are typically grown only in vivo. Low passage PDX may better model the original patient's tumor by retaining tumor heterogeneity, gene expression and similar response to treatment. PDX are thus thought to be more translational as a drug development tool. Clinical history may be available for some PDX. For example, tumors from patients who have previously been treated with a first line therapeutic may be selected to study a new drug designed for use after first line treatment failure. According to Hui Gao, a Novartis researcher, "PDX mice are so powerful because they can capture the genetic heterogeneity that exists within one patient's tumor and across many patients' tumors...That combination holds enormous promise for our ability to predict up front which drugs might help which patients."What are the drawbacks of PDX?
Setting up PDX studies can be more logistically complicated and expensive. With cell line xenografts, it's relatively simple to have 100 nude mice engrafted with HeLa cells ready to go on a Monday for screening. This can be more challenging with PDX models. PDX models may not be available for all tumor types of interest. Some PDX models are slow growing, increasing experimental time and cost. Additionally, access to PDX models can be an issue.Which mouse strains and stocks are appropriate PDX hosts?
Many of the early publications describing PDX generation referenced use of nude and scid animals as hosts. Some PDX developers continue to use those strains and stocks, but there seems to be a shift among many PDX researchers towards use of more immunodeficient strains. Strains with greater immunodeficiency such as the NOD scid or the CIEA NOG mouse® can provide better take rates in some situations. A more immunodeficient host may also better preserve tumor heterogeneity. In some cases, this tumor heterogeneity includes donor immune cells, which can cause some complications.See our Insight on this topic. More immunodeficient hosts may also show faster tumor growth compared to less immunodeficient hosts.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)