Causes of NASH in Humans
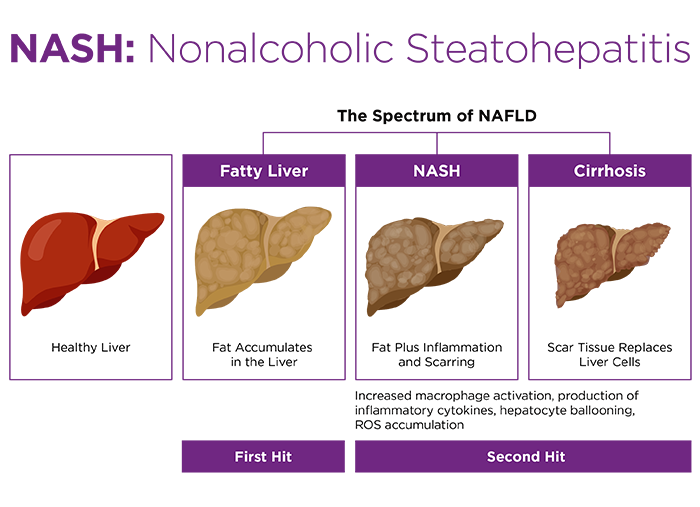
Nonalcoholic fatty liver disease (NAFLD) affects 30-40% of US adults, with approximately 3 million cases per year1. 10-15% of NAFLD patients progress to NASH, which is characterized by liver cirrhosis and hepato-cellularcarcinoma2.The pathogenesis of NASH generally follows two-steps:
- The "first hit" is accumulation fat in the liver; and
- Lipotoxicity triggers the "second hit" characterized by increased oxidative stress which results in secretion of pro-inflammatory cytokines, expression of lipogenic genes, and mitochondrial dysfunction, leading to liver cirrhosis, inflammation and ultimately liver fibrosis3,4 (Figure 1).
 It is still not clear why some people are more prone to develop NASH, though research suggests that certain health conditions make humans more likely to develop NAFLD or NASH. Below are some of such conditions:
It is still not clear why some people are more prone to develop NASH, though research suggests that certain health conditions make humans more likely to develop NAFLD or NASH. Below are some of such conditions: - Obesity
- Insulin Resistance
- Glucose Intolerance
- Type 2 Diabetes
- High Cholesterol and High Triglycerides
- Metabolic Syndrome
NASH Animal Models
Understanding the pathophysiology and treatment of NASH in humans has been limited by the lack of satisfactory genetically engineered or diet-induced mouse models which fully reproduce the key physiological, metabolic, histologic, transcriptomic, and cell-signaling changes seen in humans with progressive NASH.To date, no single animal model has encompassed the full spectrum of human disease progression, but they can imitate characteristics of the disease.
Below is the list of various genetically modified mice models that exhibit histological evidence of steatohepatitis5-7.
| Murine Models to Study NASH | Impact on NASH Development |
| ob/ob mice | These mice carry a spontaneous mutation in the leptin gene. They are hyperphagic, inactive, extremely obese, and severely diabetic, with marked hyperinsulinemia and hyperglycemia. ob/ob mice do not spontaneously progress from steatosis to steatohepatitis, requiring a 'second hit' to be administered to trigger progression to steatohepatitis. |
| aP2-nSREBP-1c transgenic mice | These mice express a truncated, constitutively active form of the SREBP-1c transcription factor. They are also hyperphagic and have massive fat accumulation in peripheral tissues with hyperglycemia and hyperinsulinemia. Other phenotypes include liver steatosis, profound insulin resistance, and increased levels of triglyceride. |
| Phosphatase and tensin homolog KO (Pten-/-) mice | Hepatocyte-specific PTEN-deficient mice spontaneously develop steatosis, steatohepatitis, and hepatocellular carcinoma by 40 weeks of age. |
| Methionine adenosyl transferase 1A KO (Mat 1A -/-) mice | Deficiency of MAT 1A results in the development of steatosis, NASH, and hepatocellular carcinoma (HCC). |
- Pten - Model 7968 - cKO
- Special Diet Administration
- Diet Induced Obese (DIO) B6
- Black 6 (B6NTac)
- Diet Induced NASH B6
Diet-Induced NASH Mouse Models
Studies have shown that under different dietary conditions, male C57BL/6 mice develop the most expressive necrosis and inflammation compared to other strains8. Some of the most practiced dietary research models are listed below.| Dietary Models | Impact on NASH Development |
| High Fat Diet (HFD) | High-fat feeding when used as a model for NASH produces variable results with respect to the levels of steatosis, inflammation and fibrosis that is dependent on the rodent species, fat content, duration of treatment, and qualitative composition of dietary fat. |
| High Sucrose and Fructose Diet | C57BL/6 mice fed with a high-carbohydrate diet for long-term (16 weeks) induces typical hepatic steatosis. For the feeding period employed, the liver does not display markers of hepatitis (inflammation, necrosis and others). |
| Methionine- and Choline-Deficient-Diet (MCD) | MCD is frequently utilized to establish NASH in the rodent liver. The lack of methionine and choline increases oxidative stress which impairs the transport of fat from the liver, leading to hepatic steatosis. This dietary model does not mimic human NASH progression because rodents exposed to MCD diets are not obese but rather they lose weight and become more insulin sensitive. |
| Diet Containing Trans-Fatty Acid | Intake of diet rich in trans-fatty acid influences liver fatty acid synthesis in humans and this is considered one of the key contributors in the development of NASH9. Similar to humans, elevated intake of trans fatty acids promote metabolic changes in mice, including proatherogenic plasma lipid profiles, hepatomegaly due to fat accumulation and inflammatory NASH-like lesions, and impaired glucose tolerance. |
| High-fat, high-cholesterol and high-fructose diet | Studies have shown that C57BL/6 mice fed a high trans-fat, high fructose, high cholesterol diet are a favorable dietary murine model to study NASH, as mice become obese, get fatty liver and develop liver inflammation and fibrosis after 26+ weeks on diet, with inter-animal variability observed for development of the inflammation and fibrosis phenotype10. Similarly, high-fat, high-cholesterol and high-fructose diet (Western Diet)-fed Ossabaw swine developed profound histological NASH with fibrosis. The study further indicates that the consumption of Western Diet causes severe microbiome dysbiosis in gut and decreased species richness/diversity which is evident in human obesity and in NAFLD/NASH patients11. |
| Mixed Models | Studies have shown that genetically modified mouse models fed with NASH inducible diet have progression of NASH similar to humans. For example, when feeding a diet rich in trans-fat, LDLr-/- mice develop atherosclerosis, weight gain, and other features of metabolic syndrome such as hyper triglyceridemia, decreased HDL, hyperinsulinemia, and insulin resistance similar to human NASH. |















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)





