Production of Human Immune System Engrafted NOG mice
In concept, the process to engraft a human immune system onto the CIEA NOG mouse®is fairly straightforward:- Introduce purified human hematopoietic stem cells into irradiated, juvenile NOG mice via tail vein injection.
- Wait 12 weeks for immune cell engraftment.
- Determine overall level of human immune cell engraftment by flow cytometry.
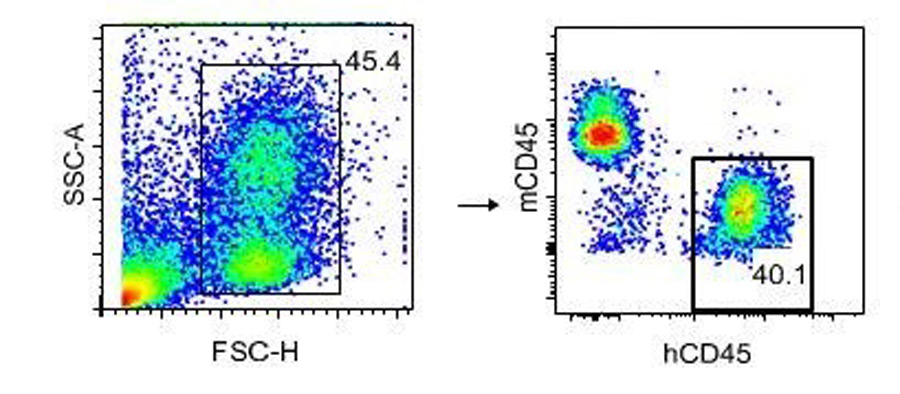
Figure 1: Flow cytometry gating scheme. Peripheral blood is collected at 12 weeks post engraftment with human CD34+ HSCs and stained with antibodies to mouse and human CD45. Lymphocytes and granulocytes are gated by forward, and side scatter as shown, and the relative percent of human cells determined as hCD45+, mCD45-.
In practice, there are many technical challenges to reliable production. Yet with well-defined production processes, it is possible to compare multiple engraftment runs and assess the impact of donor variability.In the first step, HSCs purified by para-magnetic enrichment are positively selected for the cell surface glycoprotein CD34. In addition to selection of CD34+ cells, a lineage depletion step to remove other non-HSC subsets, including T cells, is performed. Upon engraftment, the mice are maintained for 12 weeks while the human HSCs differentiate into subsets of immune cell lineages. At 12 weeks post engraftment, peripheral blood is collected and prepared for flow cytometry validation to determine the relative percent of mouse vs. human cells. An example of the flow cytometry gating strategy used to determine engraftment efficiency is shown in figure 1.
huNOG production consistency
Figure 2 shows a random sampling of 12 human immune system engrafted NOG mouse lots produced at various points over the last 20 months representing 849 huNOG mice. Each lot is engrafted with HSCs from a single donor, and carried through to flow cytometry characterization as shown in figure 1. Mice are successfully engrafted if the peripheral blood is comprised of greater than or equal to 25% human CD45+ cells. This standard was established for two important reasons. First, at least 25% positive human cells is sufficient for nearly all applications of immune system engrafted NOG mice in discovery immunology, and immuno-oncology applications, the two largest research segments utilizing these mice. Learn more about immune system engrafted mice in immuno-oncology by downloading our white paper titled Xenograft Host Selection: Important Factors for Experimental Success. Secondly, the 12 week time point is just one time point on the continuum of engraftment. Notably, mice that reach 25% or greater at week 12 typically reach a stable level ranging from 30-50% human cell reconstitution levels by 16 weeks. The 12 week time point for analysis is chosen because it is the earliest in production that one can determine the majority of mice meeting the ≥25% hCD45+ standard. huNOG mice have a typical life span of up to 1 year post engraftment and although human cell reconstitution levels typically plateau at 16 weeks, they generally maintain the week 16 levels with some incremental increases over time.Among the huNOG lots shown in figure 2, one lot completely failed to engraft (red arrow). When failures occur, it usually results in zero mice passing the engraftment threshold, and thus the entire lot is considered a failed run. The absolute reasons for failed lots are often unknown, but possible reasons include poor viability of HSCs or an unidentified factor intrinsic to the donor.
Donor-related engraftment variability
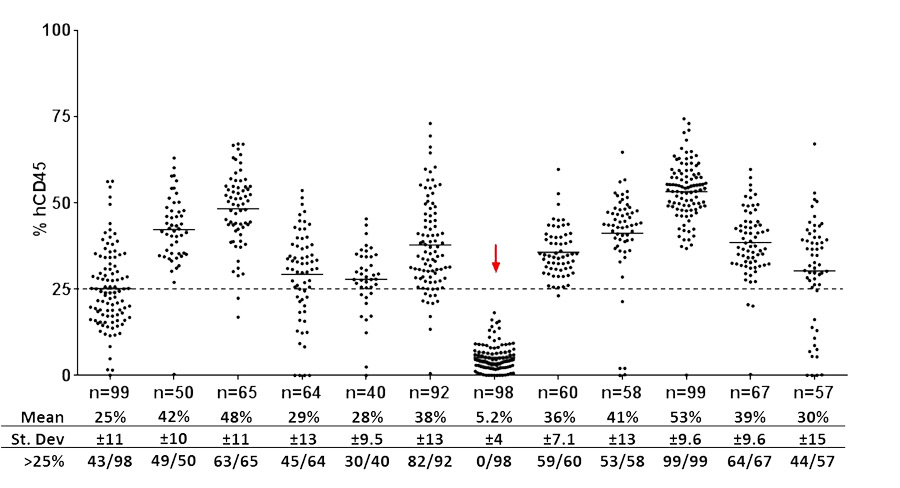
Figure 2: Engraftment results for 12 different huNOG production runs. Each series represents a different lot of huNOG production mice, measured for the relative level of human cell reconstitution at week 12 post engraftment. Each point represents one mouse and the total number of mice per lot is shown.
For the majority of lots the average engraftment is 37%, indicating that the process of huNOG engraftment at Taconic is consistent and reliable. Another reflection of quality in technical processes is the frequency a given donor lot yields successfully engrafted (≥25% hCD45+) mice. If the failed lot is removed from the data presented in figure 2, the average donor lot yields 84% successfully engrafted mice (631 successful/751 engrafted).In summary, while lot to lot variability is evident to an extent, the success of Taconic's human immune system engrafted models production program is strictly dependent on adherence to a refined and standardized process. By emphasizing operational efficiency and rigorous technical standards, the donor HSC contribution to overall variability in minimized and consistent production of engrafted mice is achieved. To learn more about the immune system engrafted models program at Taconic or to order huNOG mice today, contact Taconic at info@taconic.com or 1-888-Taconic / +45 70 23 04 05 (Europe).















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)




