Numerous strains of mice will develop obesity, metabolic dysfunction-associated steatotic liver disease (MASLD), and metabolic dysfunction-associated steatohepatitis (MASH, formerly known as NASH) when fed a "Western" diet enriched in saturated or trans fats, cholesterol, glucose, and fructose. This triggers weight gain, hepatic steatosis, and lipotoxic inflammation, leading to liver fibrosis and dysfunction. In many ways this mimics the etiology and progression of the spectrum of human disease.
Obesity and its resulting comorbidities largely arise from an improper balance between energy intake and energy expenditure, with too much of the former and too little of the latter. In rodent models, the researcher overtly enhances energy intake by offering dietary formulations that are calorically enriched relative to conventional rodent chow. On the other hand, energy expenditure is rarely manipulated by the researcher. In fact, it is commonly assumed that rodents living in close confinement and relieved from the rigors of foraging, reproduction, and predator evasion should, by default, present a low expenditure state.
This preconception overlooks a crucial disparity between humans and rodents living in a shared environment: their abilities to thermoregulate. Mice and humans maintain similar body temperatures (Tb) of about 36°C to 38°C. However, mice are roughly 3000 times smaller by mass. Smaller animals have a larger surface area to volume ratio and lose heat more rapidly to their environment.
Thermoneutrality
An animal's thermoneutral zone (TNZ) is the ambient temperature range where it can maintain basal metabolic rate (BMR). For (clothed) humans, the lower end of the TNZ is about 21°C, and this is where we like to set the thermostat: at home, in the lab, and in the vivarium. Most of the time, human Tb is sustained by metabolic heat byproduct, and heat dissipation is actually a more pressing focus. In contrast, a mouse at 21°C dedicates roughly half of its total energy expenditure toward maintenance of Tb, and it exhibits 2.5X the metabolic rate of a human1.
Mouse TNZ spans higher temperatures. In fact, mice demonstrate an interesting propensity: they adjust Tb from 36°C up to 38°C along the ambient range of 29°C to 34°C in order to keep energy expenditure, and thus BMR, stable. In part, this reflects the relative lack of heat dissipation mechanisms in rodents compared to humans. However, at temperatures below approximately 29°C, mice deploy a facultative strategy to generate heat for maintenance of healthy Tb2.
Nonshivering thermogenesis
Mammals store fat in two types of adipose tissue: white (WAT), which is chiefly used for energy reserves, and brown (BAT), which is dedicated to generating heat3. BAT is activated by epinephrine signaling through the sympathetic nervous system in response to cold stress stimuli. This initiates lipolysis of triglyceride droplets, liberating fatty acids for β-oxidation within the abundant mitochondria of BAT adipocytes.
BAT is the predominant tissue of residence for the mitochondrial membrane transporter uncoupling protein 1 (UCP1, aka thermogenin). UCP1 permeabilizes the inner mitochondrial membrane to protons, resulting in mitochondrial combustion of triglycerides without ATP production. Instead, energy is released as heat, a phenomenon termed nonshivering thermogenesis.
BAT is a crucial factor in mouse energy expenditure, as demonstrated using transgenic toxigene mice that can rendered BAT deficient in response to toxin administration4. Mice without BAT became obese and presented hallmarks of metabolic syndrome, including hyperglycemia and hypertriglyceridemia. Though they developed hyperphagia at advanced ages, obesity initially proceeded in the absence of excessive food consumption.
Modeling metabolism at thermoneutrality
Since BAT dysfunction can cause obesity, it is reasonable to infer that BAT inactivity might also enhance obesity and its comorbidities. Most vivaria maintain an ambient temperature within the human TNZ, forcing rodents to engage in frequent or constitutive nonshivering thermogenesis to maintain Tb. This has profound consequences on energy homeostasis and physiology. Mice at 30°C have roughly half the energy expenditure of mice at 22°C (kcal/h)5. This is almost entirely attributable to diminished cold-induced thermogenesis as opposed to changes in BMR or physical activity. Importantly, food intake (kcal/h) is decreased, but not as much as two-fold, at the thermoneutral temperature.
In addition to lowered energy expenditure, mice housed within their TNZ have lower heart rates and blood pressure, show reduced catecholamine and corticosteroid signaling, and generate higher levels of inflammatory cytokines6-8. For mice fed a standard chow diet, these trends do not contribute to substantial pathologies or even enhance weight gain. For mice fed a Western diet, housing at thermoneutrality clearly exacerbates metabolic disease. They accrue more WAT, have higher levels of circulating LDL cholesterol and fatty acids. They even develop atherosclerotic lesions, a disease hallmark that is otherwise poorly modeled in wild type mice.
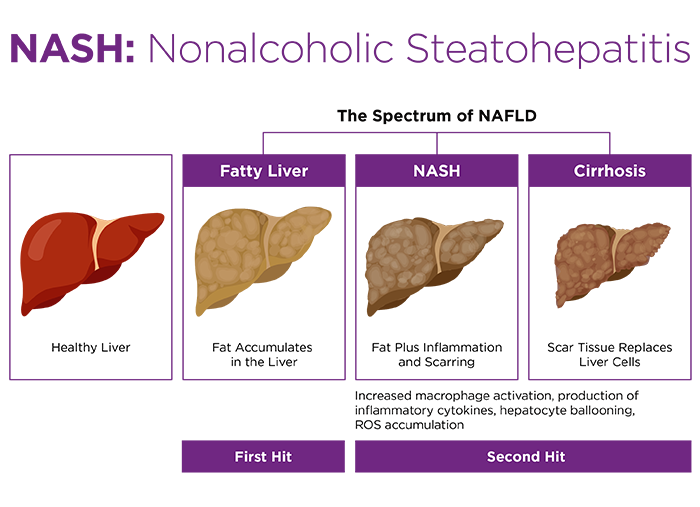
Thermoneutral housing exacerbates liver disease
Dietary MASLD and MASH are also enhanced at thermoneutrality. In a key study by Giles et al., mice were maintained on a simple high fat diet (HFD, 60% kcal fat) for 24 weeks at 22°C or 30°C7. HFD mice housed within the TNZ developed larger, more highly steatotic livers, characterized by macrophage infiltration and activation, higher pathological MASLD scores, and serum biomarker-confirmed hepatic dysfunction. Remarkably, the TNZ also enabled female HFD mice to become robustly adipose, steatotic, and hepatically compromised. Dietary obesity and MASLD models are otherwise notoriously sex biased, with females being precluded due to lack of metabolic and inflammatory susceptibilities9,10.
In addition to demonstrating reductions to energy expenditure and BAT activation within TNZ-housed mice, the researchers documented crucial alterations to their immune responses. HFD mice housed at 30°C were more sensitive to innate immune stimuli. Administration of bacterial endotoxin triggered higher production of inflammatory cytokines interleukin-6 and tumor necrosis factor-α.
Why is this pertinent? The researchers observed that the combination of HFD and thermoneutrality leads to increased intestinal leakiness, resulting in septic delivery of gut bacteria to the liver, along with their inflammatory molecules. Endotoxin is one of several characterized "second hits" that may accompany the "first hit" of steatosis to activate the immune cells that progress MASLD along the disease spectrum toward MASH11. Furthermore, thermoneutrality resulted in dysbiosis within the mouse gut microbiome, promoting expansion of Gram-negative Bacteroidetes, which are associated with MASH in humans as well as in mice11,12. The combination of dysbiosis, gut permeabilization, and enhanced immune responsiveness layered onto diminished BAT lipid oxidation appear to be an optimal formula for fatty liver disease.
Thermoneutral housing can also exacerbate liver fibrosis in non-metabolic models. Nga et al. deployed the carbon tetrachloride toxicity model at thermoneutrality13. Mice exhibited increased hepatocyte injury, infiltration of immune cells, activation of profibrotic hepatic stellate cells, and collagen deposition within the liver.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)




.jpg)

.jpg)



