Amylin liver NASH model
The Amylin liver NASH (AMLN) model was described by Trevaskis et al. in 2012 as a diet-induced model which displayed both aspects of NASH (nonalcoholic steatohepatitis) and metabolic syndrome. The authors compared two diets high in fat, fructose and cholesterol: one version with trans fat (Research Diets D09100301) and the other with lard, and concluded that "the addition of trans-fat better mirrored pathophysiological features of NASH (e.g., hepatomegaly, hepatic lipid, and fibrosis)." In both Lepob/Lepob and C57BL/6J mice, the high trans fat diet induced elevated plasma ALT and hepatic steatosis after 8 weeks (Lepob/Lepob) or 12 weeks (C57BL/6J) exposure, with both experiencing increased liver weight relative to body weight. After just 4 weeks exposure to the high trans fat diet, the Lepob/Lepob mice displayed fibrosis; in contrast, minimal fibrosis was seen in C57BL/6J mice exposed to the same diet for 16 weeks1. Further studies extended the time on diet to 12 weeks (Lepob/Lepob) or 26 weeks (C57BL/6J) and used liver biopsy to confirm development of steatosis and fibrosis prior to intervention, with both strains displaying steatosis and fibrosis at the indicated time point2.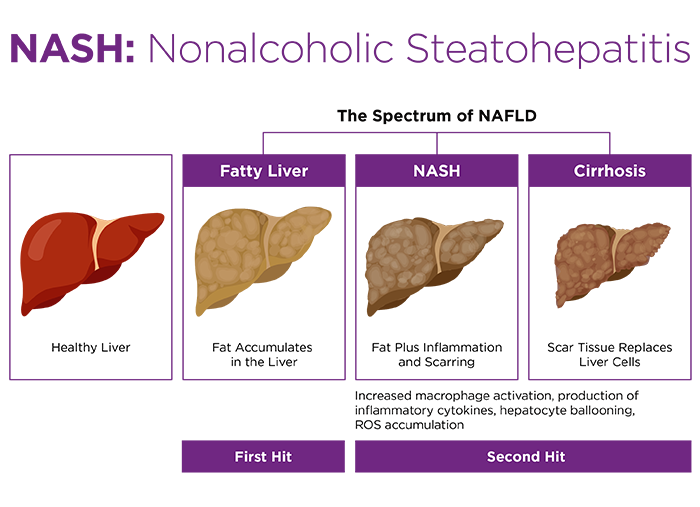 The AMLN diet (D09100301) used with either the Lepob/Lepob or C57BL/6J strain was thus adopted for drug studies directed at NASH therapeutic development by many researchers, in part due to its clinically translatable etiology. However, the unintended consequences of a decision by the US Food and Drug Administration (FDA) resulted in confusion and disarray among preclinical NASH scientists.
The AMLN diet (D09100301) used with either the Lepob/Lepob or C57BL/6J strain was thus adopted for drug studies directed at NASH therapeutic development by many researchers, in part due to its clinically translatable etiology. However, the unintended consequences of a decision by the US Food and Drug Administration (FDA) resulted in confusion and disarray among preclinical NASH scientists.
FDA ban on trans fats impacts preclinical research
Trans fats are unsaturated fatty acids with trans rather than cis configurations. Although trans fats are found naturally in meat and dairy, a significant source of trans fat in human diets has been in the form of partially hydrogenated oils, which are made industrially via hydrogenation of vegetable oils to generate a product which is solid at room temperature. Numerous studies have shown negative health impacts associated with consumption of trans fats, with increases in mortality due to all causes, as well as coronary heart disease, sudden cardiac death and fatal cancers3.With mounting evidence that the consumption of trans fats has a negative overall impact on human health, regulatory bodies made changes designed to limit consumption of artificially produced trans fats in the human diet. The FDA reclassified partially hydrogenated oils as not Generally Recognized as Safe (GRAS) in 2015 and banned use of them for most food applications as of June 18, 2018, with a complete phase out of partially hydrogenated oils in other food applications by January 1, 20214. This ban impacted the availability of Primex, a shortening containing trans fats which was a key component of the AMLN diet (D09100301).
Primex ceased being used in laboratory animal diet formulations in 2017. While many researchers stockpiled diets containing Primex prior to the discontinuation of those diets, it provided only a short-term solution. Alternative diets were required for future research endeavors. Various fat sources considered for alternative diets included: partially hydrogenated corn oil, Primex Z (a solid shortening with no trans fats) and palm oil.
"That was a challenging time for scientists studying diet-induced NASH models. It wasn't clear what the optimal replacement for the Primex diet would be," according to Dr. Matthew Ricci, Vice President and Science Director at Research Diets. "We worked with our customers to evaluate various options, including diets with different fat sources which matched the Primex diet for palmitic acid levels, trans fat levels or both. In the end, the feedback we received from most labs was that D09100310, which contains palm oil, gave similar results compared to the old Primex diet. We also think this diet may be more relevant to current human nutrition. Because of the regulatory environment, most humans are not currently eating high levels of trans fat. The original Primex shortening was replaced with a non-trans fat shortening containing a mixture of palm oil and hydrogenated soybean oil."















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)





