Due to time constraints, many of the questions submitted during the webinar went unanswered. We present a full Q&A here.
Dextran Sulfate Sodium (DSS)-Induced Colitis Models
Q: How do the acute and chronic DSS models differ?
Information on the acute model was presented in the webinar. In the chronic model, there are a number of different changes that occur. In particular, this effects the epithelium (mostly of the distal colon), with characteristic changes due to repeated injury and longer periods of regeneration. It is possible to see long term changes that can be considered dysplastic, which might lead to cancerous types of lesions.
The chronic model can have utility, but there are challenges. There are a lot of variables, including how long DSS is administered and at what frequency. This can lead to variation in response to the agent.
The advantages of chronic colitis animals models are similar to those for the acute model: it can be used with mice of any genetic background, and it is relatively easy and inexpensive. Where chronic DSS models may have less experimental utility is that they model repeated injury and not chronic inflammation itself.
Q: Regarding strains, do you have any comments on using the Swiss Webster mice for the DSS model?
As an outbred strain, Swiss Webster mice are genetically heterogeneous. While this may increase variability in the results, using Swiss Webster mice may provide information on the effect of therapeutics within a more genetically-diverse population.
Il10 Knockout Mice
Q: Which antibiotics work to ameliorate disease in Il10-/- mice?
Q: Given that weight loss isn't a characteristic of the Il10 knockout model, how can disease severity be monitored in this model?
Colonoscopy may be used to monitor disease progression, but this is time consuming and requires specialized equipment. Another method which can be used is monitoring barrier function by administering various tracers and looking at absorption of those in the blood.
One facile method which is becoming popular is to measure the amount of a molecule called lipocalin 2 in the feces. Lipocalin 2 is a product secreted from neutrophils and will be detected in the feces following ulceration or neutrophil transepithelial migration that occurs in colitis. Fecal pellets are collected, extracted, and lipocalin-2 measured by ELISA. This is a very sensitive measure of the amount of inflammation ongoing in the colon and has been validated as a quantifiable marker in several models of intestinal inflammation (PLoS One. 2012;7(9):e44328). A clinical equivalent would be fecal calprotectin assays.
Q: What about the Il10-/- and piroxicam model of accelerated disease onset?
The dosage and formulation of piroxicam, as well as the age and microbial status of the mice, need to be carefully validated for this model to be successful and reproducible.
Q: Is the Il10 knockout model appropriate for studies of Treg regulation?
Much of the research specific to Tregs has been done in adoptive transfer models. It may be worthwhile to extend these studies to other types of colitis models. DSS is probably not an ideal model to study Treg function, given that T cells play a secondary role in this model; the haptenizing agents such as oxazolone or TNBS might have some utility, with the caveat that those models have an altered cytokine profile which is more Th2 skewed.
Adoptive Transfer Colitis Models
Q: What is the success rate of disease development in adoptive transfer models?
Success depends upon several factors — the most important being cell viability and purity — and proper injection techniques. Proper gating for CD45RBhi in flow sorting is essential, and options include additional gating for CD25- and CD62L+ cells. Viability of >90% should be confirmed prior to injection.
Intraperitoneal injections of sorted cells are common; however, intravenous injections may increase success rate and consistency. Recipient mice should not be maintained on antibiotics since gut flora are critical in activating donor T cells.
Study Design
Q: How do you make results from colitis models more dependable, less variable, and more consistent over time?
Further, you should account for how the microbiome of those mice will change once they arrive in your institution. This may involve highly controlled housing and other husbandry considerations. You can minimize the effect of microbiome on assay performance by cohousing mice or exchanging bedding between control and experimental mice.
Another aspect is strain background. There is a growing realization that substrains of inbred mice can be very different. There are differences between C57BL/6 substrains which can impact study performance, particularly for inflammation models, yet references in this field rarely specify which substrain was used in a study. The field needs to recognize substrain differences, standardize selection of colitis models, and understand how these differences impact research.
Q: What models are best for testing of probiotic effects of bacteria?
Probiotics can also be evaluated in other chemically-induced models, such as oxazolone or TNBS.
Q: What is the best model for studying the role of integrins?
This model has been used extensively to determine how different integrins regulate intestinal lymphocyte homing and retention. Blocking α4β7 integrin is effective in the adoptive transfer model. Integrins have also been studied in the DSS, TNBS, cotton-top tamarin, and SAMP1/YitFc models.
Q: How important is maternal / litter effect within the various colitis models?
In general, breeding pairs which have been separated for extended periods of time will tend to develop unique microbial profiles, due to drift of commensal populations. There are several methods to mitigate this issue if using littermates in your experiment is not feasible, including co-housing or the exchange of dirty bedding prior to study initiation.
Q: Can quantifiable animal behavior be used to monitor disease progression?
There are data that changes in stereotypical mouse behaviors can be used to monitor disease progression in the DSS colitis model. In one report, the authors used the Time to Integrate to Nest Test to quantify behavioral changes that correlated with disease activity (PLoS One. 2015 Dec 4;10(12):e0143824). In another, altered burrowing behavior also showed promising results (Lab Anim. 2013 Oct;47(4):274-83).
To our knowledge, behavioral tests have not been validated with other colitis models, but these novel approaches may become valuable tools in colitis studies.
Translational Relevance of Colitis Models
Q: How were the genes known to be associated with IBD discovered?
In this presentation we reported that there were 215 known susceptibility loci; however a very recent paper has identified 26 additional loci, bring the total number of IBD-associated loci to 241 (Nat Genet. 2017 Feb;49(2):256-261). Clearly this is a very active field of investigation, and mechanistic studies are required to understand how different genetic variants influence IBD.
Q: What is the most clinically relevant colitis model?
Each model has utility for different aspects of IBD. The DSS model is very good for looking at ulceration, and repair leads to remission. In contrast, the Il10-/- model is excellent model for studying how microbiome interactions cause chronic changes in disease.
There is no single model that is most clinically relevant. One must look at the specific disease mechanisms and look at these as tools to probe how a specific mechanism influences inflammation, ulceration, and dysplasia in the GI tract. Think of the models in terms of mechanistic modeling rather than as a model that can model all disease aspects in all patients.
Q: How do animal models of colitis respond to treatment and is this response clinically relevant?
For example, the Il10 knockout model is very microbiome-driven. It responds very well to antibiotic treatment, as well as to blockade of different cytokines. Anti-TNF has a very good effect in the Il10 knockout model for preventing disease, but is not very effective at treating disease once it has developed.
If a specific therapeutic agent works in one model, this does not necessarily mean it will work in another. You can't use a single model in isolation to evaluate a therapeutic. You must evaluate a therapeutic in a number of different models using hypothesis-based objectives.
For example, if you are studying how immune cells traffic to the gut via blocking integrins, the DSS model may not be most appropriate as it drives disease directly by injury to the epithelium. Choice of model will depend on the specific mechanism of action of the proposed therapeutic agent.
Q: What effect sizes are clinically or biologically relevant in the different models as they relate to therapeutics?
In general, small changes in disease severity in colitis models may not be clinically meaningful, even if results are statistically-significant. It is well established that IBD patients are heterogeneous and respond differently to even the most effective therapeutics. Therefore, an important aspect to consider is whether therapeutic effects are shared between several colitis models in animals of different genetic backgrounds.
Q: Please comment on the failure of anti-IL17 therapy in the clinic. This therapy had positive data in preclinical models.
A similar situation occurred with IL10. This was identified as protective against colitis in mice from the Il10 knockout, but anti-IL10 therapy performed poorly in humans. In many of those clinical trials, there were not inclusion criteria to identify the subset of patients which might have responded.
We need to understand the models and refine them, so we can best understand which of them give us most predictive power as we look to develop therapeutics.
Q: Are non-human primate colitis models more clinically relevant than rodent models?
Publications in the cotton-top tamarin model show that it responds to sulfasalazine, anti-TNF, and anti-α4β7 integrin. The use of NHP models must be justifiable in accordance with the 3Rs principles (replacement, reduction, and refinement).
Q: Other than the models you presented, what are some other IBD models with potential clinical relevance?
Some of these are listed below. Please see references for more detail:
- Oxazolone (Nat Protoc. 2007;2(3):541-6)
- TNBS (2,4,6-Trinitrobenzenesulfonic acid) (Nat Protoc. 2007;2(3):541-6)
- Mdr1a-/- (Am J Pathol. 2002 Feb;160(2):739-51)
- Tbet-/-Rag2-/- (Cell. 2007 Oct 5;131(1):33-45)
- Tlr5-/- (J Clin Invest. 2007 Dec;117(12):3909-21)
- TnfΔARE (Immunity. 1999 Mar;10(3):387-98)
- SAMP1/YitFc (Inflamm Bowel Dis. 2011 Dec;17(12):2566-84)
- Citrobacter rodentium (J Immunol Methods. 2015 Jun;421:61-72)
Poll Results of Webinar Attendees
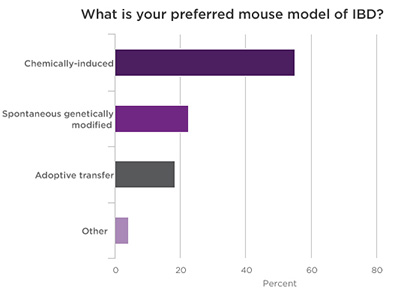
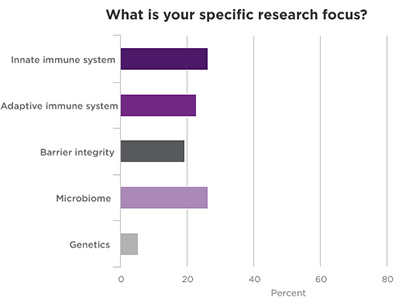















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)




