 On June 8, Dr. Kenneth Klingenberg Barfod — of the National Research Centre for the Working Environment, Denmark — presented a webinar on the importance of the murine lung microbiome, how the lung microbiome in mice can be manipulated experimentally, and its applications in disease modeling.
On June 8, Dr. Kenneth Klingenberg Barfod — of the National Research Centre for the Working Environment, Denmark — presented a webinar on the importance of the murine lung microbiome, how the lung microbiome in mice can be manipulated experimentally, and its applications in disease modeling. Compared to the extensively studied gut microbiome, the lung microbiome is a niche research area with relatively few published reports. A common — mistaken — assumption has been that healthy lungs are sterile, though no modern studies to date have shown absence of microorganisms in the lungs1. While challenges exist in sampling from the lungs, the presence of a lung microbiome in healthy humans, and mice, is now considered a fact.
Dr. Barfod discussed how the presence of microbes in healthy lungs is a balance between immigration and elimination.
- Microbes immigrate by microaspiration from oropharynx or the stomach, inhalation, and mucosal dispersion.
- We eliminate microbes by coughing, clearance by the mucosal cilia, and through the adaptive and innate immune system present at the mucosal surface of the lungs2.
The Lung Microbiome in Health and Disease
The composition of the lung microbiome has been correlated to various human diseases: asthma, acute respiratory distress syndrome, chronic obstructive pulmonary disease, HIV, and cystic fibrosis.In the webinar, Dr. Barfod explained how the lung microbiome is expected to be under the influence of many of the same factors known to impact the gut microbiome: Mode of delivery, antibiotics, breast vs. formula feeding, diet, infection and vaccination history, genetics, environmental impact such as pollution, vitamin D levels due to sun exposure, and more. All these factors may shape the immune system of the lungs, either directly or through alteration of the lung microbiome.
The gut microbiota is the largest and most diverse community of the human microbiome, and therefore perceived as the larger influence on human health relative to the lung microbiome. However, in relation to the risk of lung diseases specifically, Dr. Barfod theorized that the lung microbiome in early life may be a more important determiner than the gut microbiome (Figure 1), but that this may be opposite later in life.
Mice are relevant animal models for investigating causality of the lung microbiome in disease. Dr. Barfod emphasized the need for establishing actual, casual relationships for moving beyond the observed correlations between human disease and the lung microbiome.
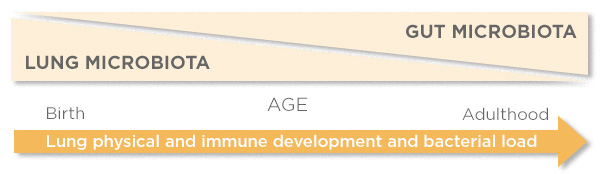
Figure 1: Theoretical Importance of Microbiota. Courtesy of K.K. Barfod.
In this regard, animal models were highlighted as crucial. The in vivo approach can be used to gain mechanistic insight through manipulations of the lung microbiome with antibiotics, probiotics, pulmonary medicines, or by creating genetically modified animals to mimic human diseases and conditions. Additionally, animal models allow for sampling approaches not possible in humans.
The research contributions of Dr. Barfod and his colleagues have shown that there is an actual microbial community in the lungs of mice, not just contamination by or a reflection of the gut microbiota3,4, and that the murine lung microbiome may be manipulated by intranasal antibiotic treatment3. This vindicates mice as relevant models in lung microbiome research.
Microbiome Study Design Factors
Dr. Barfod highlighted the myriad of factors that may affect and potentially bias animal studies. As a scientist, it is essential to know if your study subjects are independent of each other for choosing the proper analytical and statistical approaches. Some of the things you would want to know are:- Origin (vendor or breeding facility)
- Changes in diet and other husbandry factors from vendor/breeding facility to your lab
- Are your mice from the same cage? From the same room? From the same mother?
- Are they the exact same age?
As differences in the lung microbiota has been observed between male and female mice3,4,5, he also put forward the relevance of including both sexes in the experiment.
Sampling and Studying the Murine Lung Microbiome
– Dr. Barfod
Several helpful tips were given during the webinar, such as performing analysis on sequencing data with and without subtraction of OTUs found in blank control samples. Dr. Barfod shared with the audience the observation that contamination could drive clustering of the samples, and that the clustering disappeared after removing the OTUs from the blank controls.
Also, technical advice for sampling of sterile bronchoalveolar lavage (BAL) fluid was given at the webinar, such as bleeding mice before BAL to obtain more fluid. Ligation of one lung lobe was mentioned as a means to obtain more experimental endpoints; i.e. one lobe can used for BAL and the other for other purposes, such as looking for tissue-dwelling microbes.
Dr. Barfod highlighted that the BAL procedure can easily be implemented as an add-on in gut microbiome studies to enable more experimental endpoints and increase discovery in the research field. Using both "ends" of the animals — looking at the lung as well as the gut microbiome — is meaningful from a harms reduction perspective, as more questions can then be answered using the same number of animals.
Germ-free mice were mentioned as a feasible way to manipulate the microbiota and dissect importance of specific organism or communities; e.g. through inoculation with probiotic strains or human microbiotas.
Murine Lung Microbiome Q&A
 The webinar ended with questions from the audience. There wasn't time to address all questions during the webinar, so we present them here with Dr. Barfod's answers.
The webinar ended with questions from the audience. There wasn't time to address all questions during the webinar, so we present them here with Dr. Barfod's answers.
Q: Are there any publications looking at the effects of lung microbiota on lung immune system?
Q: Can microbiota transfer from gut to lung? And how?
Q: Can you separate the host DNA from the bacterial DNA?
Q: Is there a difference in tissue interface for microbiota (mucosa layer), as has been seen in cecal mucosa in gut?
Q: How do you account for cell or mucus associated bacteria using BAL?
Q: How would you study biofilms in your model?
Q: If there is little overlap from the gut microbiome to the lung, can the lung microbiome transfer to gut?
Q: In terms of a gut-lung axis, is there a microbiome in the blood in the naive murines?
Q: Is there a way to specifically deplete microbiota from the lung without touching the gut microbiome? What about germ free mice?
Q: How do you isolate a lung lobe prior to lavage and do so in a sterile manner?















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)



