Source: Exp Anim. 2014; 63(3): 289-296
Super immunodeficient hosts: NOG and NSG Strains
The NOG and NSG strains are considered super immunodeficient mouse models. The combination of strain background, inclusion of the scid mutation, and a targeted disruption of the IL2 receptor common gamma chain results in a loss of T, B and NK cells, as well as complement C5 deficiency, and additional deficiencies in macrophage and dendritic cell function. As these strains are on the NOD inbred background, they also carry a particular allele of Sirpα which promotes enhanced engraftment of human hematopoietic cells and tissues. These characteristics make the NOG and NSG superior strains for engraftment of difficult cancer cell lines and patient-derived tumors, as well as for human immune system engraftment. These are very useful tools, but as with all tools, a clear understanding of their strengths as well as their limitations is crucial.Development of human lymphomas in super immunodeficient hosts
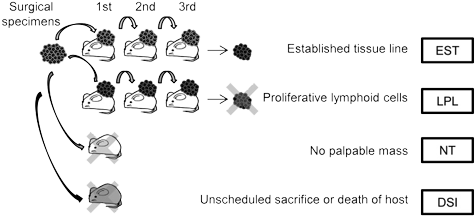
Several groups have recently published on a poorly understood aspect of PDX development. Patient-derived tumor models start by implanting a tissue section from a surgical resection of a patient's tumor. That tumor tissue is heterogeneous, and will include tumor cells of various types and may also include human immune cells. Because NOG and NSG mice are so efficient at engrafting human immune cells, some proportion of original engrafted human tumors may be replaced with lymphoid cells. These lymphoproliferative tissues arise from human immune cells in the implanted tumor, particularly in Epstein-Barr virus positive donors. This finding was observed across a range of tumor types and across varying implantation protocols. For some tumor types, this process may be the greatest cause of tumor model development failure.Mechanism of lymphoproliferative lesion replacement of human tumor
The Epstein-Barr virus is associated with lymphoproliferative disorders in humans. EBV is one of the most common human viruses, occurring in >90% of the population. In immunocompetent humans, EBV is typically kept in check by the human immune system. However, in immunocompromised humans, EBV can cause lymphoproliferation, particularly of B cells. A similar mechanism may be at work when lymphoproliferative lesions are seen in development of patient-derived xenograft models in super immunocompromised mouse strains such as NOG or NSG.Researchers from Chugai, Pharma Logicals and CIEA examined lymphoproliferative lesions that arose during development of colorectal cancer PDX and found that the lesions contained multiple types of lymphoid cells. When lyphoproliferative lesions were present, the lymphoid cells totally replaced the original tumor cells in most cases. In some mice where lymphoid tissues replaced the tumor cells, lymphoproliferative lesions were also found in other tissues such as spleen, liver, kidney and lung1.
Prostate cancer PDX are difficult to establish and may display long latency until growth. Researchers from University Hospital Basel, Oncotest, University of Bern and Claraspital Bern in Switzerland used NOG and NSG mice to develop subcutaneous and subrenal prostate cancer PDX lines and experienced human donor lymphoma development, particularly in the mice which showed early mass formation. Of the subset of mice which formed a tumor by three months post-transplantation, 80% were identified as human donor-derived lymphomas. In this study, the lymphomas were primarily EBV+ diffuse large B cell lymphomas, though one T cell lymphoma was also observed2.
Researchers from KU Leuven and University Hospitals of Leuven identified a number of different post-transplant disorders in NOG mice transplanted with metastatic human melanomas, but at relatively low frequency (12%). In this study, affected mice presented with dermatitis and alopecia, wasting and poor tumor engraftment which was caused by a systemic immunoinflammatory disorder similar to xenogeneic Graft versus Host Disorder (GvHD) involving human donor immune cells. Xenografted mice which presented as clinically healthy did not display any human lymphoid cell infiltrates into various mouse tissues or the xenografted tumor. Variation was observed among F1 expansion groups receiving tissues from the same donor, with some displaying successful tumor engraftment and others suffering post-transplant disorders3.
Methods to reduce lymphoproliferative complications
One challenge posed by the prospect of lymphoproliferative lesion replacement of tumor tissues is that palpable masses form both when a tumor engrafts successfully as well as when a lymphoproliferative lesion replaces the tumor tissues. This makes monitoring of graft size by palpation an unreliable indicator of graft success. The group from Chugai, Pharma Logicals and CIEA identified splenomegaly as a good indicator of lymphoproliferative lesions. This indicator may help scientists working to develop new PDX models to choose the most appropriate specimens to take into further passages. Another method suggested by the group from Chugai, Pharma Logicals and CIEA is the administration of a B cell-depleting antibody such as rituximab. Initial data indicates this may be a promising method to reduce lymphoproliferative lesions during PDX development.The Swiss researchers highlighted that use of mouse embryonic mesenchyme (MEM) as a method to reduce donor lymphoma development is an open question to be further investigated. For slower growing tumor types, those mice which develop tumors quickly might be considered suspect and should be examined for lymphoma development.
For PDX strategies which utilize cell suspensions, one method to examine might be selection to remove donor immune cells prior to transplantation. Similar methods are employed to remove mature T cells from hematopoietic stem cell sources prior to immune system humanization in order to prevent GvHD. This type of selection might reduce the type of GvHD-like disorders seen by the KU Leuven group.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)





