| Model No. | Nomenclature | Genotype |
|---|---|---|
| APOE-F | B6.129P2-Apoetm1Unc N11 | ko/ko |
| APOE-M | B6.129P2-Apoetm1Unc N11 | ko/ko |
ApoE

- Description
- Data
- Price & Licensing
- Health Report
- Overview
- Genetics
- Guides & Publications
- Applications & Therapeutic Areas
- Transit, Housing & Welfare
- Diet
Overview
Nomenclature: B6.129P2-Apoetm1Unc N11
- Contains a disruption of the endogenous murine apolipoprotein E (ApoE) gene
- ApoE, a glycoprotein, is a structural component of very low density lipoprotein (vLDL) synthesized by the liver and intestinally synthesized chylomicrons
- ApoE is also a constituent of a subclass of high density of lipoproteins (HDL) involved in cholesterol transport
- ApoE mediates high affinity binding of chylomicrons and vLDL particles to the LDL receptor, allowing for specific uptake of these particles by the liver, preventing the accumulation of cholesterol rich particles in the plasma
- Homozygous ApoE mice are devoid of apoE protein
- Mice develop normally, but exhibit five times normal serum plasma cholesterol and spontaneous atherosclerotic lesions
- Studies indicate a role for ApoE in immune system regulation, nerve regeneration, and muscle differentiation
- Useful in studying the role of apoE in lipid metabolism, atherogenesis, and nerve injury and to investigate intervention therapies that modify the atherogenic process
- This line carries the Gpi1a allele and the ES1a and ES1b alleles in contrast to the C57BL/6N inbred which is Gpi1b and ES1a
- Approximately 0.6% of mice in our APOE colony exhibit malocclusion. Animals with malocclusion are euthanized. Links between atherosclerosis and periodontal disease have been reported in humans and it is possible that these mice develop periodontal disease that leads to inflammation, gum disease, and subsequently malocclusion. To reduce the incidence of malocclusion, Taconic recommends providing animals with autoclavable chew sticks
Origin
Genetics
Guides & Publications
Initial Publication: Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. (1992) Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA, 89(10):4471-5.
Applications & Therapeutic Areas
- "Alzheimers Disease"
- Autoimmune Disease
- Cardiovascular Disease
- Diabetes
- Metabolic Disease
- Microbiome
- Neuroscience
- Psychiatric Disorders
Transit, Housing & Welfare
Need more info? Click the live chat button or Contact Us
Packing Practices
Taconic standard practice is to recombine animals of different home cages and/or ages from a single model and sex during packing, except in specific cases where Taconic's animal welfare policy prohibits recombination due to aggression or other concerns. When an order is fulfilled with animals from more than one week of birth, this standard practice results in animals from a range of birth weeks packed together in a single TTC. When an order is fulfilled with animals from genotyped models, this standard practice results in animals from different home cages packed together in a single TTC.
Customers who wish to keep animals from different weeks of birth separated should place orders with the special instruction "Divide and label by age." Note that this special request can result in increased costs for additional Taconic Transit Cages, dividers and/or freight charges.
Taconic discourages other types of custom packing requests as they can have a negative impact on animal welfare. Learn more.
Diet
Data
Download phenotypic data for Apoe-M mice.
Histologic Examination of Taconic B6.129P2-Apoetm1Unc N11 Knockout Mice
Frimorfo of Fribourg, Switzerland performed a phenotypic analysis on Taconic B6.129P2-Apoetm1Unc N11 (Apoe Knockout Mice) using the Morphome Discovery Program. Three approximately 10 week old Apoe Knockout Mice (2 females and 1 male) and 3 age matched control C57BL/6NTac Mice (2 females and 1 male) were analyzed.
Two Apoe Knockout Mice (ID F018-01 and F019-01) had mild to moderate atherosclerotic lesions in various body regions. The locations and stage of the lesions were consistent with previous reports in the published literature. (Reddick RL, Zhang SH, Maeda N. (1994) Atherosclerosis in mice lacking apoE. Evaluation of lesional development and progression. Arterioscler Thromb., 1994 Jan;14(1):141-7) Only major arteries were evaluated and characteristic lesions were noted in the two females, but not the male animal. All lesions were mild to moderate in severity and consisted of intimal plaques, adventitial fibrosis, and occasional inflammatory foci of the vascular wall. Severe lesions with vascular occlusion or aneurysm formation were not seen in this age group. No additional model-specific histological lesions were observed.
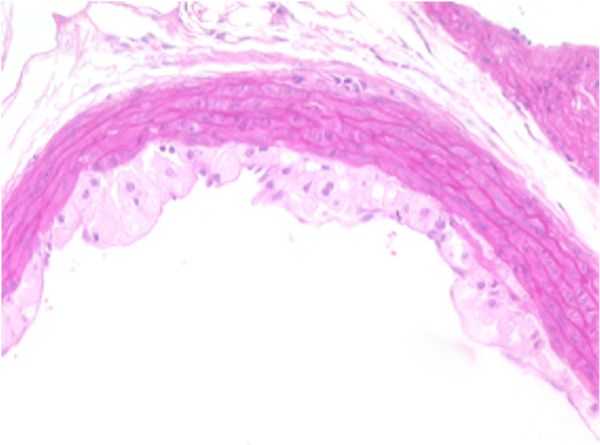
AORTA–a: Stain: Elastic Fiber. F19-01
Early Atherosclerotic plaque formation, tunica intima, thoracic aorta.
Representation of an accumulation of lipoid filled or foam cells of the xanthomatous type occupying and expanding the subendothelial space, effectively separating the endothelial cell layer from the internal elastic membrane of the tunica intima. The wall of the aorta is mildly thickened by this accumulation of material and the internal elastic membrane appears to be intact within this segment.
This response represents early atherosclerotic plaque formation localized within the tunica intima. The rest of the artery appears to be within normal limits.
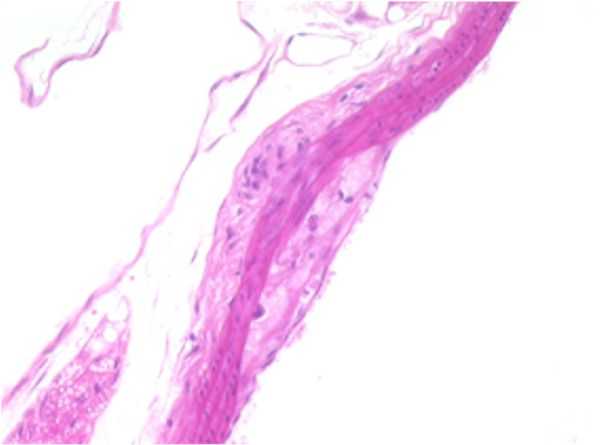
AORTA–b: F19-01
Atherosclerotic plaque, tunica intima/media, thoracic aorta.
Presented with a segment of arterial wall that exhibits a mild, focally-extensive compression of the tunica media by opposing accumulations of eosinophilic, foamy, or lipid appearing material situated within the subendothelial space of the tunica intima and the tunica adventitia. An early, minimal to mild fibroplasia is evident in these areas within both the tunica intima and tunica adventitia. The endothelium is elevated by this material occupying the subendothelial space.
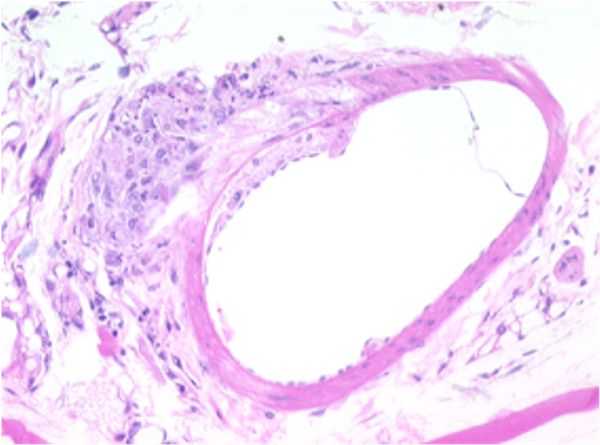
SMALL ARTERY: F18-01
Atherosclerotic plaque, tunica intima/media, thoracic aorta.
A focally-extensive area of the tunica media is moderately to markedly compressed by an accumulation of eosinophilic, lipoid, or fatty material within both the tunica intima and tunica adventitia.
The tunica adventitia also contains a focal, nodular, well-demarcated area of infiltrating cells characterized by mild numbers of macrophages, occasional lymphocytes, and increased collagen fibers.
The rest of the artery appears to be within normal limits. This area of constriction and corresponding proliferation may represent an area of early plaque formation.
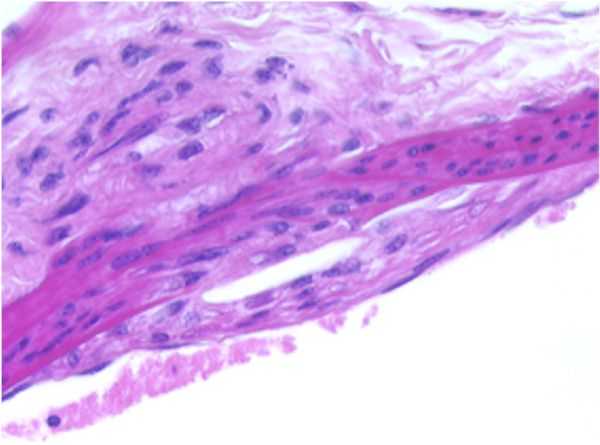
THORACIC AORTA: F18-01
Atherosclerotic plaque, tunica intima/media, thoracic aorta.
A focally-extensive area of the endothelium appears to be elevated off the tunica media by an accumulation of eosinophilic, lipoid, or foamy appearing material which occupies the subendothelial space between the tunica intima and the tunica media. There appears to be a focally-extensive disruption of the internal elastic membrane by the accumulation of eosinophilic, lipoid or foamy appearing material within the subendothelial space between the tunica intima and the tunica media.
An area of similar material accumulation also appears present opposite the sub-endothelial proliferation. The clear, vesicular spaces within the sub-endothelial material layer were considered representative of multiple, variably-sized cholesterol clefts. Also present within the area of foam cell accumulation was a mild infiltration of collagen and fibroblasts, representing early fibroplasia.
None of the arteries contain indications of either calcification or cellular necrosis, which is usually associated with atherosclerotic change in vessels. This absence may be representative of either a lack of lesion chronicity, or depicts how the lesion is presented in this animal model.
- Licensing
- Pricing - USD
- Pricing - EUR
- Pricing - USD Nonprofit
- Pricing - EUR Nonprofit
- Select my Health Standard
- Get Custom Pricing Guide
ApoE
Conditions of Use for Taconic Transgenic Models™
Taconic Transgenic Models™ (Models) are produced and distributed under rights to patents and intellectual property licensed from various institutions. Taconic sells the Models to purchasers, grants to each purchaser a right under Taconic's rights in such licensed patents and intellectual property to use the purchased Model in consideration of purchasers' acknowledgement of and agreement to the Terms and Conditions for Taconic Models, Products and Services and the following terms of use:
- Title to these Models and biological materials derived from them remains with Taconic.
- The Models will be used for research purposes only.
- The Models will not be bred or cross-bred except to obtain embryos or fetuses required for research purposes unless additional rights have been granted in writing by Taconic.
- The Models and biological materials derived from them will not be distributed to third parties or used for commercial purposes.
- Non-profit purchasers may not use this Model and/or biological materials derived from it in sponsored research or contract research studies unless it is purchased at the for-profit price.
Pricing - USD
Murine Pathogen Free (MPF) Health Standard
APOE Female
APOE-F Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | $ 200.00 |
APOE Male
APOE-M Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | $ 200.00 |
Pricing - EUR
Murine Pathogen Free (MPF) Health Standard
APOE Female
APOE-F Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 182,00 € |
APOE Male
APOE-M Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 182,00 € |
Pricing - USD Nonprofit
Murine Pathogen Free (MPF) Health Standard
APOE Female
APOE-F Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | $ 175.00 |
APOE Male
APOE-M Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | $ 175.00 |
Pricing - EUR Nonprofit
Murine Pathogen Free (MPF) Health Standard
APOE Female
APOE-F Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 159,00 € |
APOE Male
APOE-M Genotype ko/ko
Pilot-sized cohorts are readily available. Large cohort requests have a minimum 8-week lead time. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 159,00 € |
Select my Health Standard
Need help choosing the right Taconic Biosciences health standard for your research?
Use the Health Standard Selector to enter your exclusion list. The tool will tell you which health standards meet your requirements.
Get custom pricing guide
Schedule A Scientific Consultation
Speak with a PhD-level Field Application Scientist who can help you select the most appropriate model and maximize your experimental success.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)






