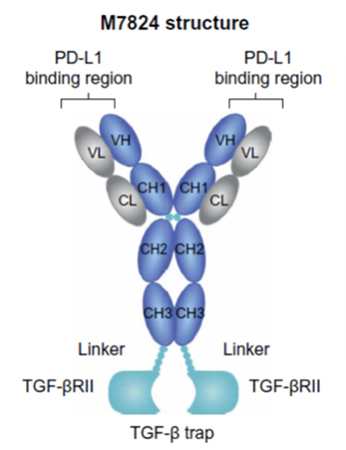
Structure of EMD Serono's bifunctional
immune-oncology therapeutic M7824, from Lan et al.3
Immunogenicity can obscure efficacy in early preclinical research
Biologic drugs such as monoclonal antibodies or RNA therapies can trigger immune reactions (immunogenicity) upon treatment. Such immune reactions can result in reduced drug efficacy as well as safety issues. This can be a problem not only in the clinic, but also in the discovery phase. Administration of monoclonal antibodies and other protein drugs can induce expression of anti-drug antibodies (ADAs) in immuno-oncology animal models. These ADAs can significantly complicate efficacy determinations by reducing exposure, neutralizing the therapeutic, or even causing anaphylaxis. This can be a major roadblock during early-stage drug discovery which confounds the identification of lead therapeutic candidates. Given that compensatory changes to reduce or eliminate immunogenicity can be further engineered onto highly efficacious candidates at later stages in the development process, use of an early screening animal model in which immunogenic responses do not occur can facilitate screening of lead molecules.Alternate strategies
Researchers have often used scid mice, which lack T and B cells, as a tool to assess in vivo efficacy in a mouse model that cannot produce neutralizing antibodies1,2. However, the severe immune deficiency of a scid background mouse may not be desirable for drug discovery programs aimed at modulating immune cell function. One example is the increased use of syngeneic tumor models for immuno-oncology research.Syngeneic tumor models are immunocompetent mice implanted with tumor cell lines derived from the same background strain. For example, the B16 melanoma tumor cell line was derived from the C57BL/6 inbred mouse strain, and B16 cells can be easily engrafted in C57BL/6 mice. The C57BL/6 host mice are immunocompetent and are therefore useful to evaluate immunotherapy approaches to engage immune cells against the tumor. If the immunotherapy being tested requires the participation of mouse T cells, a scid mouse lacking T cells is not suitable. However, the consequence of immune competence can be immunogenicity that undermines efficacy studies. In that case, a mouse model with disrupted B cell development affecting only the adaptive antibody response may be an appropriate alternative. B cell-deficient animal models permit efficacy testing of immuno-oncology (IO) therapeutics when anti-drug antibodies (ADA) would otherwise limit clinically-relevant dosing.
Immunotherapy Case Study: bintrafusp alfa
EMD Serono's immuno-oncology pipeline includes bintrafusp alfa (M7824), a bifunctional therapeutic designed to target two different pathways used by cancer cells to evade immune control. Bintrafusp alfa combines a monoclonal antibody against PD-L1 fused to a receptor fragment designed to trap TGF-β. Inhibition of PD-1 and PD-L1 has been exploited to develop multiple approved cancer drugs, including the blockbuster KEYTRUDA® (pembrolizumab). The cytokine TGF-β can promote tumor progression and metastasis as well as abet tumor immune evasion. Bintrafusp alfa combines simultaneous blockade of both the PD-L1 and TGF-β pathways.Although bintrafusp alfa is a fully human immunoglobulin G1 (IgG1), it cross-reacts with murine PD-L1. Similarly, the TGF-β trap portion of bintrafusp alfa recognizes both the human and murine cytokines. This allows preclinical studies in mouse models using the actual clinical candidate rather than a surrogate.
EMD Serono scientists overcame the immunogenicity problem through use of a mouse model incapable of producing ADAs. "Because M7824 is a recombinant human protein," they wrote, "it induces a strong immunogenic response in immunocompetent mice if dosed repeatedly for more than 6 days. Therefore, B cell-deficient mouse strains (Jh and μMt−) were used for in vivo studies to enable testing of clinically-relevant repeat dosing schedules3."
— Lan, et al.
Bintrafusp alfa also provided long-term protective immunity against tumors; when Jh mice that had been cured of EMT-6 orthotopic tumors were re-challenged with subcutaneous EMT-6 cells 218 days later, they did not develop tumors — even though no further bintrafusp alfa treatment was provided3.
Because Jh mice retain immune cells other than B cells, the researchers were able to interrogate changes to various immune cell types in the tumor models. Lan et al. found that bintrafusp alfa treatment increased the density of CD8+ tumor infiltrating lymphocytes (TILs) and tumor-infiltrating NK cells (TINKs) relative to treatment with anti-PD-L1 or TGF-β monotherapies. They also saw effects on myeloid cell populations, including dendritic cells, neutrophils, monocytes, and macrophages3.
Through cell depletion experiments, the EMD Serono researchers were able to identify CD8+ T cells and NK cells as playing major roles in the anti-tumor activity of bintrafusp alfa and hypothesized that myeloid cells may also be involved3. Supported by these observations, EMD Serono has advanced bintrafusp alfa into clinical trials against a range of tumor types4.
Jh Mouse available on two key strain backgrounds to facilitate syngeneic tumor studies
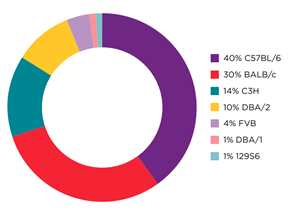
Percent Breakdown of Common Strains Used
for Syngeneic Models by Existing Murine
Tumor Cell Lines
Download the infographic to learn more about Jh mice models.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)




