Taconic Biosciences, a global leader in providing genetically engineered rodent model solutions, has launched three distinct packages of products and services utilizing the Wild Mouse Microbiome (WildR) to dramatically improve microbiome-based research outcomes. Taconic holds a license for the Wild Mouse gut microbiome from the National Institutes of Health's (NIH) National Institute of Diabetes and Digestive Kidney Diseases (NIDDK). This agreement supports an existing microbiome solutions portfolio, which includes germ-free mice and custom microbiota associations.
Taconic Biosciences Launches New Microbiome Service to Increase Translatability of Research Mice
Published: September 1, 2024
Recent studies in both humans and mice have shown that the response to treatment, like immunotherapies, may be dependent on the composition of the microbiome. However, the gut microbiome of standard laboratory mice is usually devoid of common microorganisms and may therefore not be the most replicative model for human gut microbiome profiles. In order to have a more translatable model, researchers have begun utilizing wild mouse gut microbiota in their research mice, to better mimic a human microbiome. This discrepancy between natural and laboratory microbiome profiles has notable impacts on not only microbiome studies, but also investigations targeting the immune system, including drug discovery efforts.
The Wild Mouse Microbiome (WildR) is often an excellent option for researchers who wish to increase the translatability of their study mice.
The WildR profile is fully characterized, licensed, and has been successfully validated in multiple research studies. In a paper published in Science earlier this year, mice harboring the wild mouse gut microbiota were found to be better at recapitulating the immune response and immune-related adverse events when compared to standard laboratory mice treated with a CTLA-4 immune checkpoint blockade (ICB).
Taconic Biosciences is offering three comprehensive packages with standard legal terms.
Each package includes a 3-year license for Wild Mouse Microbiome.
- Package 1: 2 vials of frozen microbiota material is shipped directly to the customer. The customer performs the association at the end-facility using germ-free mice or any other mouse strain of interest.
- Package 2: Taconic associates three breeding pairs of germ-free mice with the WildR microbiota by fecal microbiota transfer. The 3 male and 3 female WildR mice (6-12 weeks of age) are then shipped to the customer’s facility.
- Package 3: Taconic associates three breeding pairs of germ-free mice with the WildR microbiota by fecal microbiota transfer. The WildR-associated animals are then setup for breeding, pups are weaned, and PCR-based health testing is performed. The original WildR mice (18-24 weeks of age) and approximately 6-18 WildR mouse offsprings (6-12 weeks of age) are shipped to the customer. A health report is included.
“Taconic recognizes the impact of the gut microbiome on diverse biological processes,” remarks John Couse, PhD, Vice President of Scientific Services. He further notes, “The wild mouse gut microbiome has important research applications. We remain committed to improving translatability and reproducibility in all facets of preclinical research.”
Schedule A Scientific Consultation
Speak with a PhD-level Field Application Scientist who can help you select the most appropriate model and maximize your experimental success.
Taconic offers a comprehensive portfolio of products and solutions for microbiome research studies.
Connect with a microbiome expert to discuss how the wild mouse microbiome toolkit can improve your research, or call 1-888-TACONIC (888-822-6642) in the US, +45 70 23 04 05 in Europe, or email info@taconic.com.
About Taconic Biosciences, Inc.
Taconic Biosciences is a fully licensed, global leader in genetically engineered rodent models and services. Founded in 1952, Taconic provides the best animal solutions so that customers can acquire, custom-generate, breed, precondition, test, and distribute valuable research models worldwide. Specialists in genetically engineered mouse and rat models, microbiome, immuno-oncology mouse models, and integrated model design and breeding services, Taconic operates service laboratories and breeding facilities in the U.S. and Europe, maintains distributor relationships in Asia, and has global shipping capabilities to provide animal models almost anywhere in the world.
Media Contacts:
For scientific inquiries:
Lisa Goldberg, PhD
Director Scientific Program Manager
Schedule A Scientific Consultation
Speak with a PhD-level Field Application Scientist who can help you select the most appropriate model and maximize your experimental success.
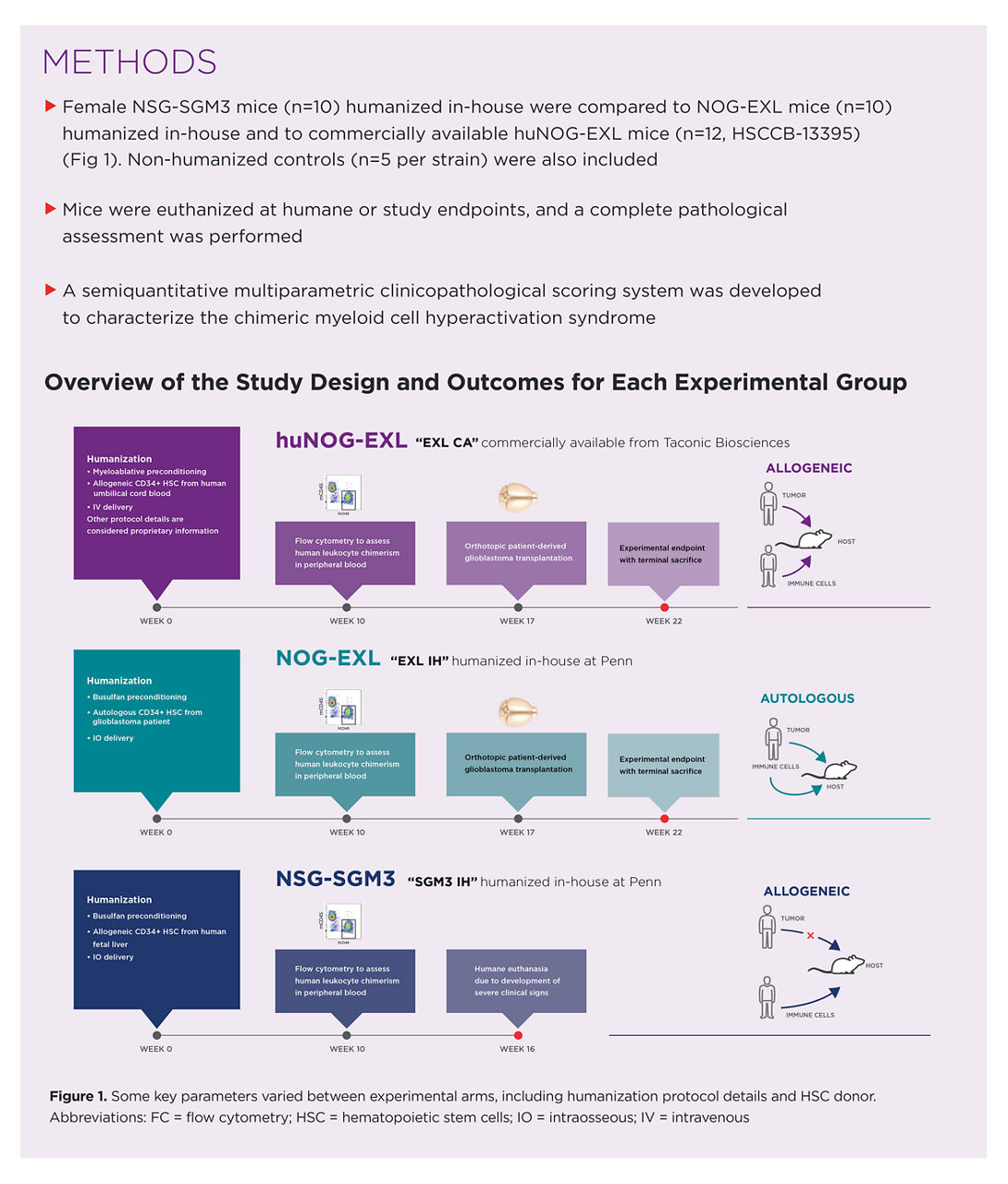
Researchers at the University of Pennsylvania (Penn) sought to recapitulate key features of glioblastoma in a mouse model following unsuccessful attempts with other models. Co-authors of the study are part of the Comparative Pathology Core and Stem Cell and Xenograft Core, and the University of Pennsylvania’s Glioblastoma Multiforme Translational Center of Excellence, which seeks to create novel therapeutics to be used in glioblastoma studies, in part by establishing disease models that recapitulate key features of disease without succumbing to severe adverse events. Previous attempts to study glioblastoma using the NSG-SGM3 mouse failed; all humanized NSG-SGM3 mice died before glioblastoma engraftment. The scientists approached Taconic with their concerns and were presented with the NOG-EXL model, which was then used in subsequent studies to determine survivability. This study also allowed researchers to characterize myeloid cell hyperactivation, a biological process that severely limits the use of mouse models in long-term studies.
Researchers analyzed:
- NSG-SGM3 mice humanized in-house at Penn with allogeneic transplants (SGM3 IH)
- NOG-EXL mice humanized in-house at Penn with autologous transplants (EXL IH)
- huNOG-EXL mice available off-the-shelf from Taconic underwent allogeneic transplants (EXL CA)
Mice from the two NOG-EXL groups reached the experimental endpoint of 22 weeks after humanization while the SGM3-IH group was unable to continue; mice were humanely euthanized at 16 weeks after humanization. Overall, the researchers noted the longer lifespans of the humanized NOG-EXL mice and much less severe adverse outcomes compared to the humanized NSG-SGM3 mice. NSG-SGM3 mice displayed signs of more severe myeloid hyperactivation syndrome (MCH), including higher levels of leukocytes and more widespread lesions in various organs, making them unsuitable for long-term glioblastoma investigations. In particular, mast cell infiltration severely affected the pancreas in mice of the NSG-SGM3 group. In the liver, severity was consistent across groups, but not lesion quality. Hepatocellular necrosis in NSG-SGM3 mice was more common. Willis et al. concluded that NOG-EXL mice are more clinically stable following humanization, regardless of being humanized in-house or commercially within Taconic.
This successful collaboration has led to several conference posters and presentations, and, most recently, a publication in Veterinary Pathology.
Schedule A Scientific Consultation
Speak with a PhD-level Field Application Scientist who can help you select the most appropriate model and maximize your experimental success.
The Taconic NOG Portfolio of superimmunodeficient and humanized immune system mice
The NOG mouse offers many advantages over standard nude and scid strains, particularly for engraftment of challenging cell lines, patient-derived xenografts, and immune system humanization.

FcResolv® NOG Portfolio
Super immunodeficient mouse models with murine Fc gamma receptors knocked out provide improved accuracy in antibody-based studies.
Researchers who work with antibody-based therapies find that murine Fc gamma receptors (FcγRs) can confound preclinical study results, causing false positives or false negatives that lead to incorrect conclusions and derail drug discovery. By knocking out these receptors, FcResolv® NOG models provide clarity in antibody-based drug studies, offering greater confidence and more translatable data while utilizing fewer resources.















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)

.jpg)


.jpg)





.jpg)

.jpg)